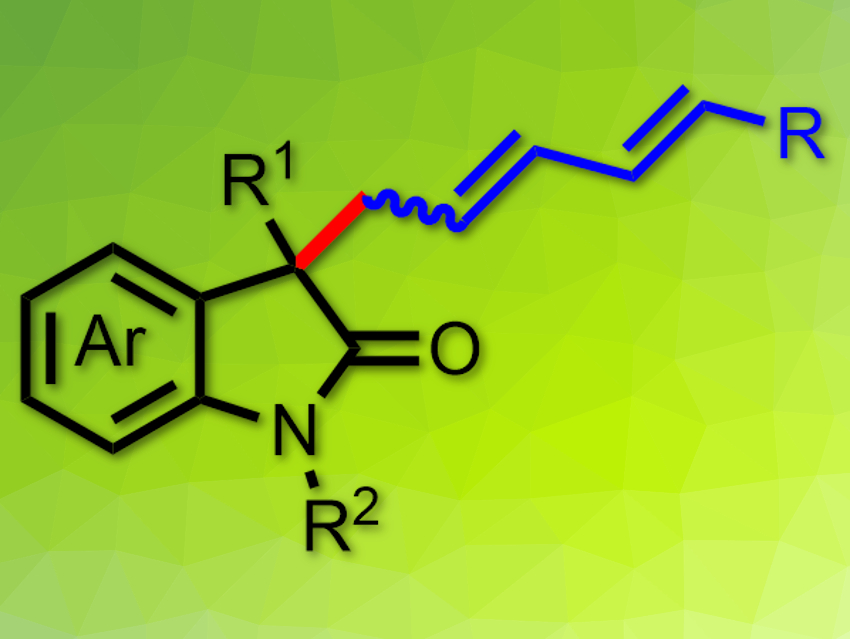
Conjugated 1,3-dienes are useful building blocks, e.g., for medicinal chemistry and natural product synthesis. Thus, efficient and atom-economical methods to access them are useful.
Chuan-Jun Lu, Qing-Bao Song, Zhejiang University of Technology, China, and colleagues have developed a palladium-catalyzed allylic alkylation of oxindoles with cyclopropyl acetylenes which gives 1,3-dienes (pictured). The team used Pd(dba)2 (bis(dibenzylideneacetone)palladium(0)) as a catalyst, PPh3 as a ligand, 3‐chlorobenzoic acid as an additive, and 1,4-dioxane as the solvent. The reaction gives good to excellent yields of the desired diene products.
The products feature a quaternary stereocenter and are formed with high regio- and stereoselectivities and good atom economy. The reaction could be compatible with enantioselective catalysis. The mechanism proposed by the researchers involves a vinyl palladium intermediate, which undergoes a β‐C elimination to form an alkylpalladium(II) intermediate. This is converted to a vinyl allene by β‐H elimination. A hydropalladation of the vinyl allene gives a π‐allylpalladium species, which isomerizes and is captured by the oxindole to give the final product.

- Palladium-Catalyzed Allylation of Cyclopropyl Acetylenes with Oxindoles to Construct 1,3-Dienes,
Chuan-Jun Lu, Xin Yu, Y-Ting Chen, Qing-Bao Song, Zhen-Ping Yang, Hong Wang,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.201901536