Highly functionalized pyrrole, pentafulvene, and pyrrolopyridine derivatives are useful compounds in the fields of medicinal and organic materials chemistry. However, synthetic methods for these derivatives are limited and often require starting materials that are difficult to obtain.
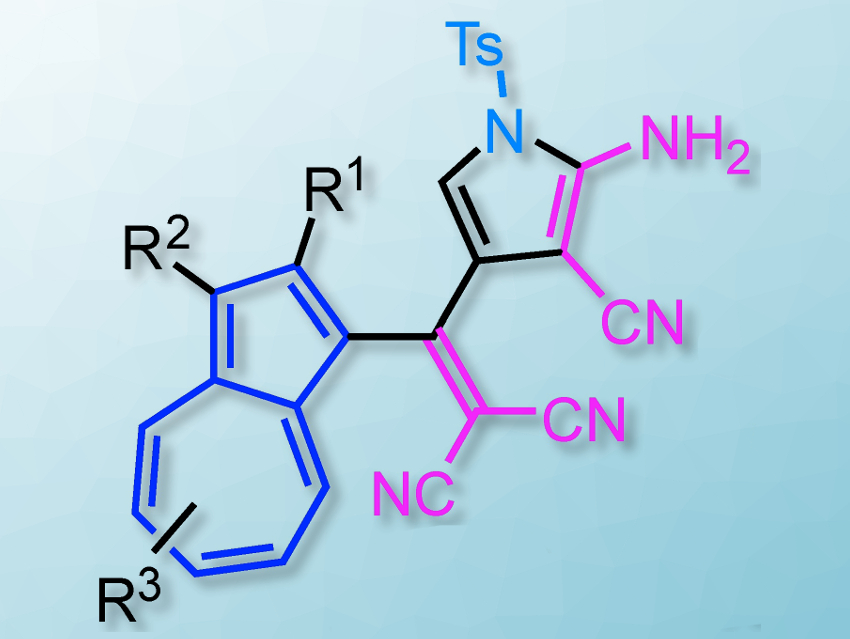
Taku Shoji, Shinshu University, Japan, and colleagues have developed a practical synthesis of highly functionalized pyrrole, pentafulvene, and pyrrolopyridine derivatives. The desired compounds are prepared via a [2+2] cycloaddition–retroelectrocyclization (CA–RE) of N-substituted propargylamines with tetracyanoethylene, followed by treatment with silica gel (pictured below). The silica gel mediates the formation of the desired ring from a tetracyanobutadiene intermediate.
The team found that the selectivity for the product of the reaction (i.e. pyrrole, pentafulvene, or pyrrolopyridine) is dependent on the substituent at the nitrogen atom of the propargylamines (see below). The products were prepared in high yields from inexpensive and readily available reagents under mild reaction conditions.

- Molecular Transformation to Pyrroles, Pentafulvenes, and Pyrrolopyridines by [2+2] Cycloaddition of Propargylamines with Tetracyanoethylene,
Taku Shoji, Sho Takagaki, Yukino Ariga, Akari Yamazaki, Mutsumi Takeuchi, Akira Ohta, Ryuta Sekiguchi, Shigeki Mori, Tetsuo Okujima, Shunji Ito,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201904926