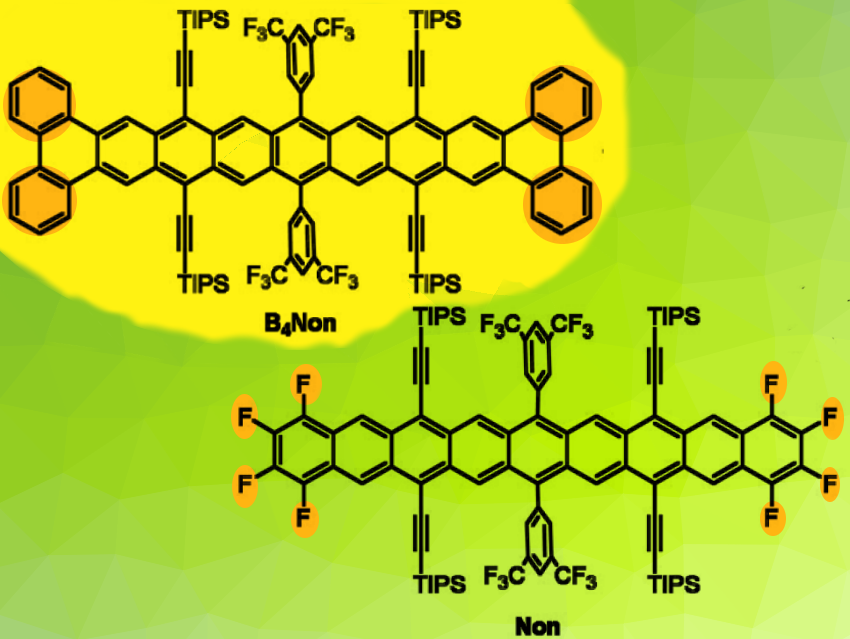
The synthesis of higher acenes is a challenge. Unsubstituted, they are both insoluble and vulnerable towards ambient conditions. Heptacenes have been stabilized by bulky silylethynyl substituents. Only very few nonacenes have been isolated and structurally characterized. An example is pictured (bottom right).
Uwe H. F. Bunz, Ruprecht-Karls-University Heidelberg, and Centre for Advanced Materials, Heidelberg, Germany, and colleagues have prepared a novel, reasonably stable tetrabenzononacene. In B4Non (pictured) the stabilization is due to the attachment of four benzo units at the cataposition of the acene unit. No heteroatom substitution at/on the aromatic backbone is needed.
The material displays sharp NMR resonances. The four benzo units blueshift the absorption spectrum in comparison to the linear nonacene Non and significantly increase the stability in the solid state.
The researchers conclude that B4Non is highly stable in the solid state. Benzo-wings therefore should allow stabilization of other, hitherto only moderately persistent, reactive aromatics.
- Tetrabenzononacene: “Butterfly Wings” Stabilize the Core,
M. M_ller, S. Maier, O. Tverskoy, F. Rominger, J. Freudenberg, U. H. F. Bunz,
Angew. Chem. Int. Ed. 2019.
https://10.1002/anie.201909614