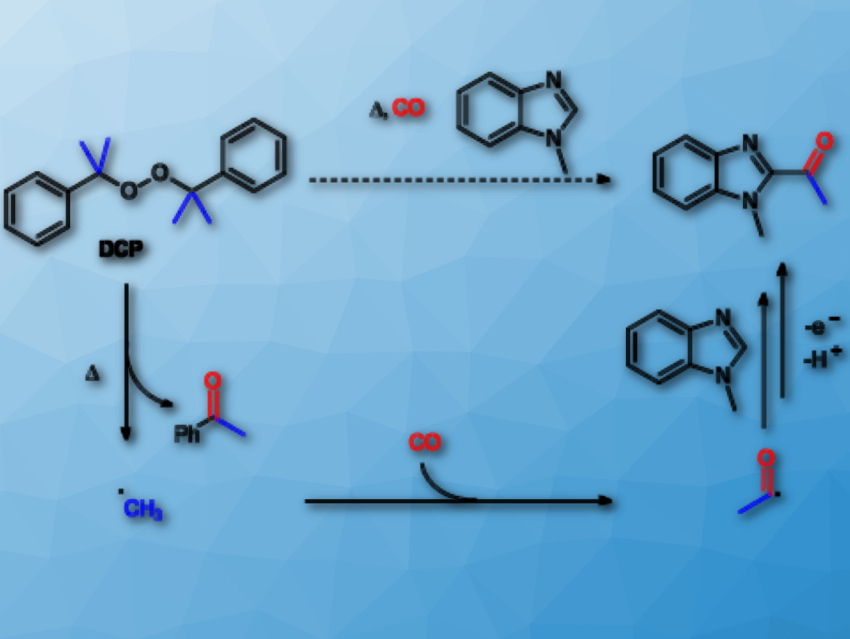
Nitrogen-containing heterocycles have wide applications in chemistry and biology. In particular, acetyl heterocycles such as 1-acetylisoquinolines, 2-acetylbenzimidazoles, and 2-acetylbenzothiazoles are attractive intermediates for the preparation of molecules with pharmacological or biological activities. Therefore, a convenient and effective method for their synthesis is useful.
Youcan Zhang, Zhiping Yin, and Xiao-Feng Wu, Leibniz-Institut für Katalyse e.V., University of Rostock, Germany, have developed a procedure for the carbonylative acetylation of heterocycles. The team selected 1-methyl-1H-benzo[d]imidazole as a model substrate. Dicumyl peroxide (DCP) acts as the oxidant and methyl source. The reaction takes place in the presence of acetic acid (HOAc) in a mixed solvent of 1,2-dichloroethane (DCE) and PhCl at 140 °C under 50 bar CO.
Concerning the reaction pathway, firstly, high temperature induces organic peroxide to produce methyl radicals (pictured). These capture CO to form acetyl radicals. Finally, acetic acid initiates the acetyl radicals and the heterocycles to undergo radical addition to give the desired products in moderate to good yields.
Isoquinolines and benzimidazoles are transformed into the corresponding 1-acetylisoquinolines and 2-acetylbenzimidazoles. In addition, 2-acetylbenzothiazoles can be obtained when using di-tert-butyl peroxide (DTBP) as the methyl source catalyzed by Mn(acac)2 in PhCF3.
- Carbonylative Acetylation of Heterocycles,
Youcan Zhang, Zhiping Yin, Xiao-Feng Wu,
European Journal of Organic Chemistry 2019.
https://doi.org/10.1002/ejoc.201901728