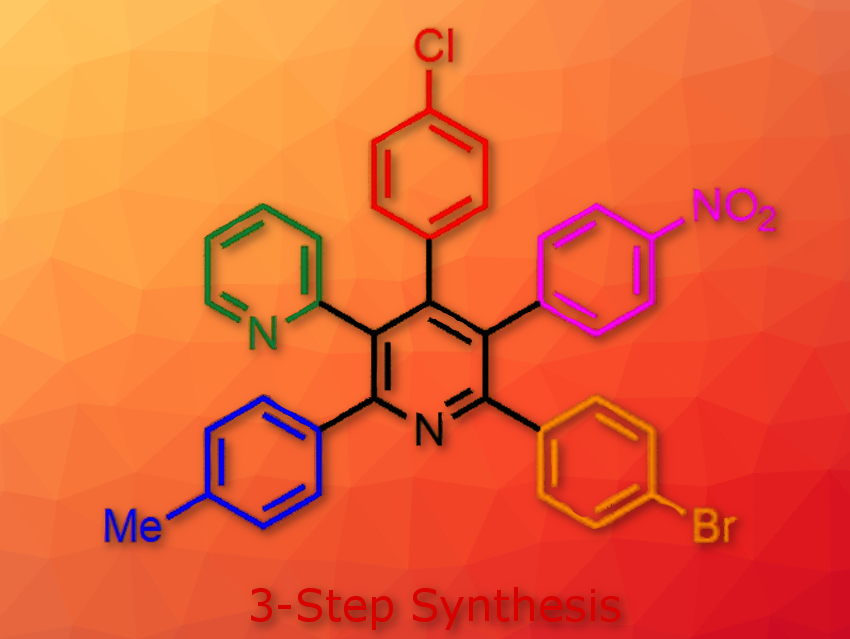
Fully and differently arylated pyridines (pictured) are challenging targets in organic synthesis. There are only a few synthetic methods including successive Suzuki-Miyaura cross-coupling reactions or ring transformation of five-membered heterocyclic compounds. However, these methods require at least seven-step reactions and sometimes suffer from control of the regioselectivity.
Nagatoshi Nishiwaki, Kochi University of Technology, Japan, and colleagues have synthesized differently substituted pentaarylpyridines in only three steps:
- Condensation of methylpyridine with benzonitrile forming β-pyridylenamine.
- Aldol condensation of benzaldehyde with β-phenylacetophenone to form an enone.
- Condensation of the enamine and the enone under microwave heating in the presence of FeCl3 under air, in which FeCl3 serves as both an acid catalyst and an oxidant for the intermediate.
This protocol facilitates the synthesis and molecular design of multiply arylated/alkylated pyridines by simple alteration of the starting materials with simple experimental manipulations. The team also synthesized polyarylated bipyridines and terpyridine using this method.
- Three Step Synthesis of Fully and Differently Arylated Pyridines,
Mao Arita, Soichi Yokoyama, Haruyasu Asahara, Nagatoshi Nishiwaki,
Europ. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901663