Diiron species can perform reactions not available to monometallic species. This is due to the cooperative effects of the two iron atoms working together. Half-sandwich organoiron complexes containing the robust cyclopentadienyl ligand have potential applications in medicinal chemistry and in catalysis. However, the classical procedures to obtain this family of compounds have some limitations when it comes to the structures of the other ligands.
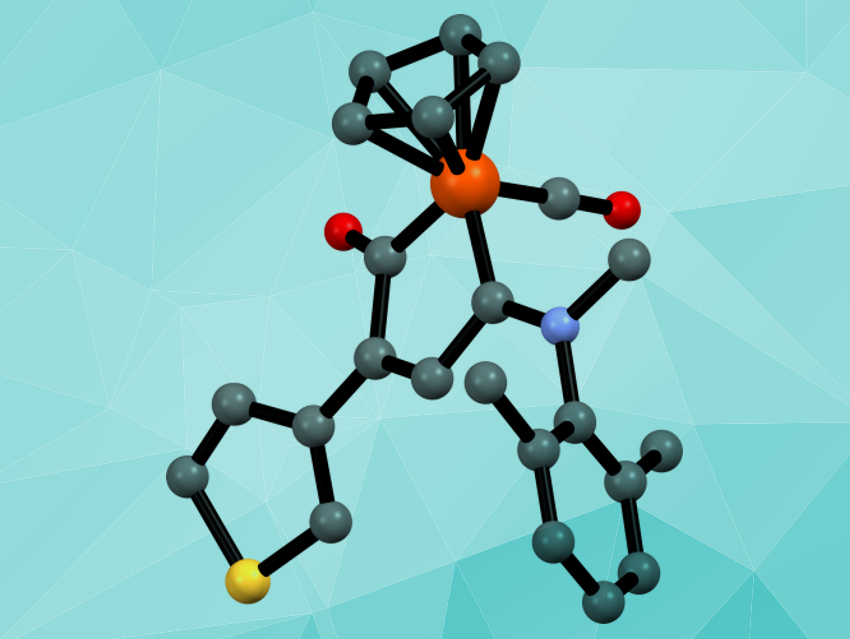
Fabio Marchetti, University of Pisa, Italy, and colleagues have developed a synthetic strategy that uses a diiron precursor to create half-sandwich iron complexes with a metallacycle that is unique in organometallic chemistry: an amino-substituted ferracyclopentenone. The metallacycle is synthesized starting from the commercially available Fe2Cp2(CO)4. This compound is converted into diiron μ‐vinyliminium complexes (pictured below on the left), which are then converted to the desired ferracyclopentenone complexes (pictured below on the right) using pyrrolidine as a reducing agent.

The reaction is straightforward and works for a range of different substitutents. The obtained compounds show significant cytotoxicity against certain cancer cell lines, in some cases with a reasonable degree of selectivity for cancer cells over non-tumor cells.
- Piano Stool Aminoalkylidene-Ferracyclopentenone Complexes from Bimetallic Precursors: Synthesis and Cytotoxicity Data,
Dalila Rocco, Lucinda K. Batchelor, Eleonora Ferretti, Stefano Zacchini, Guido Pampaloni, Paul J. Dyson, Fabio Marchetti,
ChemPlusChem 2019.
https://doi.org/10.1002/cplu.201900639