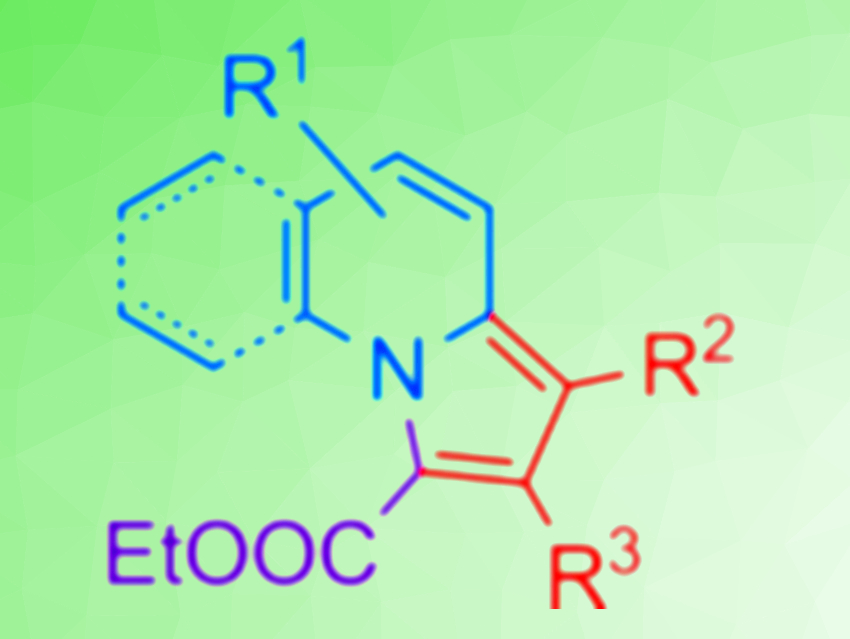
Indolizines, a class of heteroaromatic compounds, are often found in natural products and are a part of many synthetic pharmaceuticals. Thus, an environmentally friendly and efficient method to synthesize indolizines for practical applications would be useful.
Lei Wang, Zhengqiang Li, Jilin University, Changchun, China, and colleagues have developed a biocatalytic synthesis route to obtain substituted indolizines (pictured). The team used pyridine derivatives, electron-deficient alkynes (alkynoates) or alkenes (acrylates), and ethyl diazo-acetate (EDA) as the substrates. Hemoglobin was used as the catalyst and ethanol as the solvent.
EDA first reacts with hemoglobin to form an iron carbenoid. This intermediate is intercepted by pyridine to produce a pyridinium ylide. Then, a tetrahydroindolizine or dihydroindolizine is formed by adding an acrylate or alkynoate to the pyridinium ylide via a 1,3-dipolar [3+2] cycloaddition reaction. Finally, the target indolizine is produced by hemoglobin oxidation.
The reaction is environmentally friendly and efficient. It uses simple alcohols as solvents, has a non-toxic protein catalyst, proceeds under mild reaction conditions, and provides moderate to high yields of the products.
- Hemoglobin-Catalyzed Synthesis of Indolizines Under Mild Conditions,
Fengxi Li, Xuyong Tang, Yaning Xu, Chunyu Wang, Liu Zhang, Jiaxin Zhang, Jiaxu Liu, Zhengqiang Li, Lei Wang,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901591