Carbonylation reactions are important in organic chemistry. Ureas, for example, are carbonyl compounds with many applications. A commonly used carbonyl source for the synthesis of ureas is carbon monoxide, which is highly toxic, flammable, and hard to work with. Therefore, safer alternatives are needed. Metal carbonyls, for example, are good replacements for carbon monoxide: They are relatively stable and easy to handle.
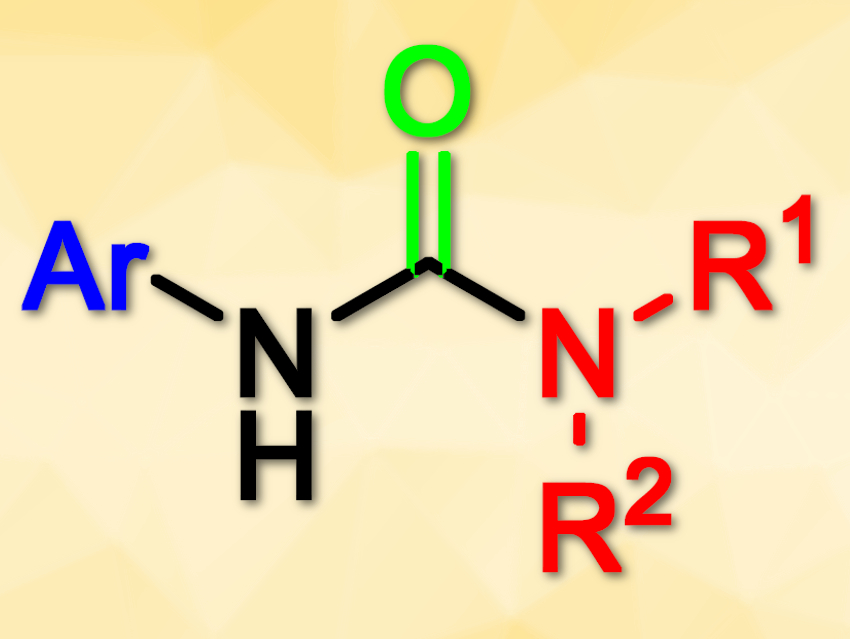
Mozhdeh Mozaffari and Najmeh Nowrouzi, Persian Gulf University, Bushehr, Iran, have found that a palladium acetate/triphenylphosphine (Pd(OAc)2/PPh3) system can catalyze the synthesis of symmetrical and unsymmetrical ureas (pictured). The reaction uses chromium hexacarbonyl, Cr(CO)6, as a convenient and safe alternative carbonyl source. Aryl iodides, sodium azide, and amines are used as the substrates. The reaction is performed in toluene at 100 °C in the presence of K2CO3 as base and gives the desired products in good to excellent yields.
The reaction proceeds via a carbonylation of the aryl iodide, followed by an anion exchange with the azide. A Curtius rearrangement of the resulting aroyl azide and a nucleophilic addition of the amine then gives the final product. The protocol is operationally simple and has a broad substrate scope.
- Palladium catalyzed synthesis of symmetrical and unsymmetrical ureas using chromium hexacarbonyl as a convenient and safe alternative carbonyl source,
Mozhdeh Mozaffari, Najmeh Nowrouzi,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901273