The detection of dangerous gases and vapors is important in both the home and in industry. Changes in the color or the fluorescence properties of a compound can offer a low-cost way to do this without a need for expensive equipment.
Tomás Torroba, University of Burgos, Spain, James D. E. T. Wilton‐Ely, Imperial College London, UK, and colleagues have synthesized ruthenium complexes with a perylenemonoimide (PMI) unit (example pictured below). These complexes can detect toxic vapors and gases in air. The fluorogenic PMI group (pictured in green) was functionalized with a terminal alkyne or pyridyl group to allow attachment to the Ru either as a vinyl or a pyridyl ligand. PMI compounds often suffer from poor solubility, so the team included a solubilizing unit with three polyethylene glycol chains (pictured in blue).
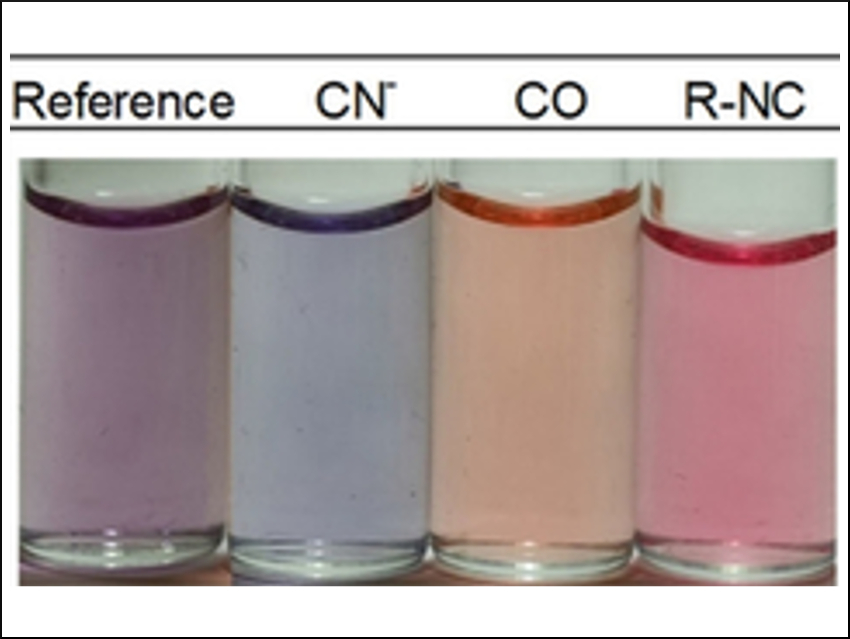
The complexes are effective sensors for carbon monoxide, cyanide, and isocyanide. They change their color and fluorescence behavior in the presence of these species. The sensors are both selective and sensitive, which the team attributes to the high affinity of the ruthenium complexes for ligands such as CO and CN–. Existing detectors based on electrochemical or semiconductor systems are often compromised by either low selectivity or sensitivity.

- Synthesis and Application of Ruthenium(II) Alkenyl Complexes with Perylene Fluorophores for the Detection of Toxic Vapours and Gases,
José García‐Calvo, Jonathan A. Robson, Tomás Torroba, James D. E. T. Wilton‐Ely,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201903303