Despite a number of successful drugs, there is still a need for improved compounds with anticancer activity. Platinum-based drugs are the most used chemotherapeutics for cancer treatment. Unfortunately, many patients face unwanted side effects and drug resistance. Ruthenium complexes with pyrithione (2-mercaptopyridine-N-oxide) ligands could be an alternative with potent anticancer activity.
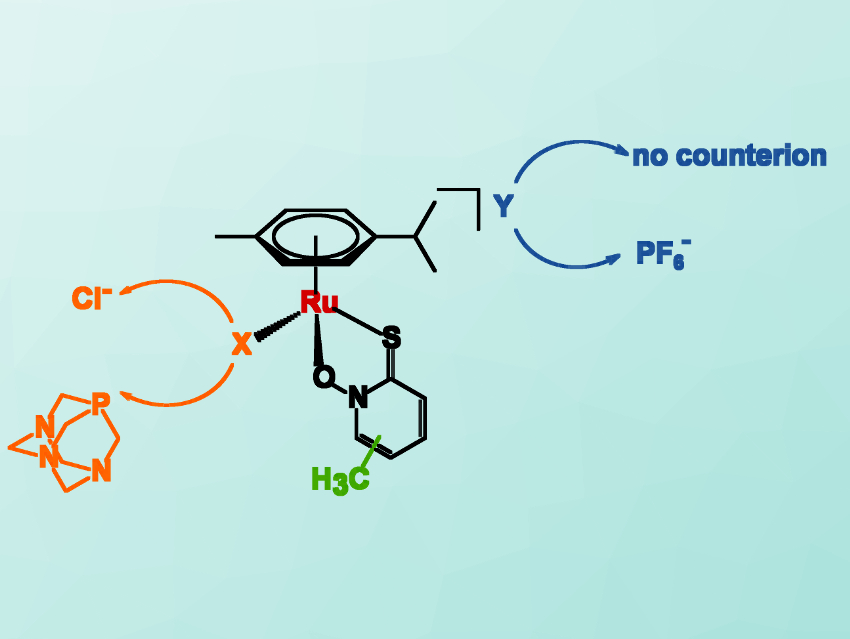
Iztok Turel, University of Ljubljana, Slovenia, and colleagues have synthesized a range of organoruthenium(II) complexes with methyl‐substituted pyrithiones and examined their anticancer activity. The team used complexes with chlorido- or 1,3,5‐triaza‐7‐phosphaadamantane (pta) ligands (pictured in orange) and pyrithiones with a methyl substituent in different positions (pictured in green). This results either in neutral complexes or in positively charged ones with a PF6– counterion (pictured in blue).
The team found that all compounds are stable enough in aqueous media as well as in human blood plasma for biological evaluation. They have similar anticancer activities in a very low micromolar range. However, the researchers found that a methyl substituent at positions in which it can increase the electron density on sulfur improves the activity. According to the team, this work could help with future drug development.
- Towards Identification of Essential Structural Elements of Organoruthenium(II)‐Pyrithionato Complexes for Anticancer Activity,
Jerneja Kladnik, Jakob Kljun, Hilke Burmeister, Ingo Ott, Isolda Romero‐Canelón, Iztok Turel,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201903109