C–O bond formations with inexpensive olefins can be used to make valuable chemicals such as alcohols, epoxides, and ethers. However, this generally requires the use of strong oxidants. Using inexpensive and environmentally friendly molecular oxygen as a terminal oxidant is a challenge for synthetic and catalytic chemists. Its electronic triplet nature hinders reactions with singlet organic molecules and when it reacts, it can be difficult to control the products.
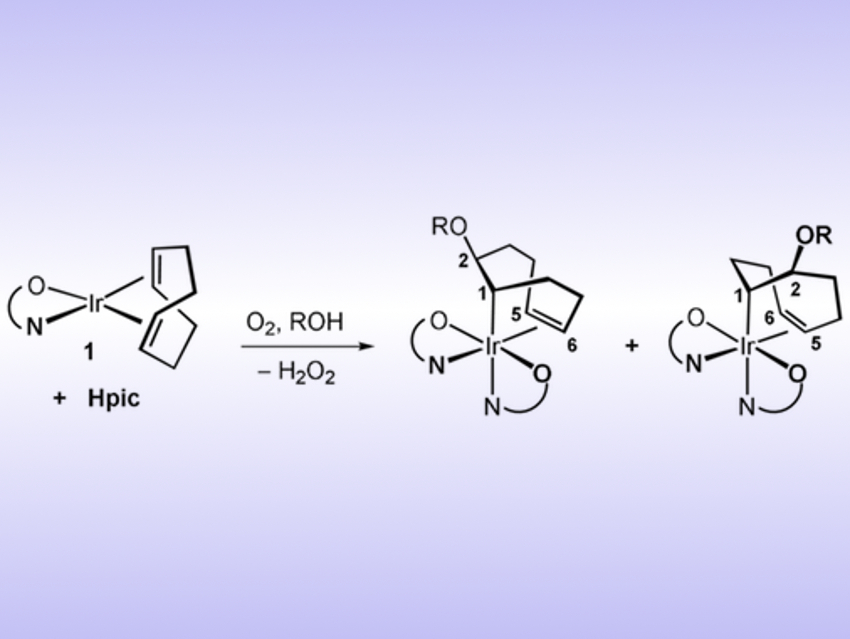
Cristina Tejel, Universidad de Zaragoza, Spain, Agustí Lledós, Universitat Autònoma de Barcelona, Spain, and colleagues have developed a way for the hydroxylation and alkoxylation of olefins coordinated to an iridium center. In this reaction, iridium(I) is oxidized to iridium(III), which makes the olefin ligand susceptible to nucleophilic attack. The complex can then react with either water or an alcohol to give HO- or RO-functionalized products (pictured). The reaction requires the presence of a coordinating protic acid such as picolinic acid, denoted as Hpic.
The method provides a straightforward path to olefin oxyfunctionalization with alcohols or water. The complex is activated by oxygen via an inner‐sphere pathway, which then promotes an outer‐sphere nucleophilic attack on the olefin. This is an unprecedented reaction in iridium chemistry. The iridium complex also allows a reversible and simple exchange of the RO groups in solution with a different alcohol (R’OH).
- Inner‐Sphere Oxygen Activation Promoting Outer‐Sphere Nucleophilic Attack on Olefins,
Paula Abril, M. Pilar Río, José A. López, Agustí Lledós, Miguel A. Ciriano, Cristina Tejel,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201903068