Stable oxide semiconductors can be used, e.g., for solar-fuel production and the photocatalytic remediation of organic pollutants. Silver-based ternary or quaternary oxide semiconductors are particularly interesting in this context because these compounds exhibit a p-type semiconductor behavior. They can be used to drive technologically important reactions such as CO2 reduction, hydrogen evolution, oxygen reduction, and so on. AgVO3, for example, has a suitable band gap to harvest visible light.
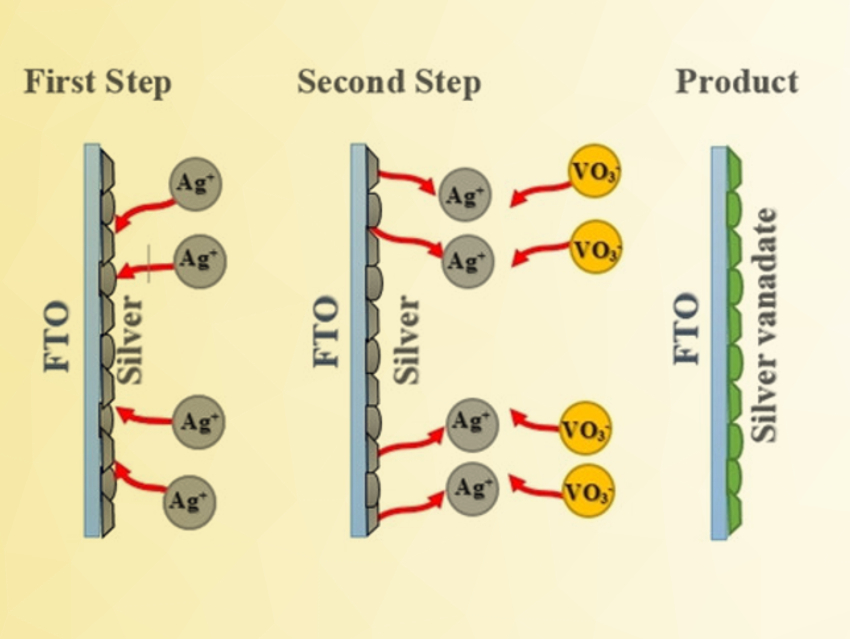
Krishnan Rajeshwar, The University of Texas at Arlington, USA, and colleagues have synthesized two polymorphs of AgVO3 (α and β). The researchers developed a two-step approach for the electrodeposition of AgVO3 thin films on a transparent and conducting oxide glass substrate (pictured). In the first step, the electrodeposition solution contained AgNO3 as a silver source, sodium dodecyl sulfate as a binder, and tetrabutylammonium perchlorate (TBAP) as a supporting electrolyte in acetonitrile. The second step was performed in an ammonium metavanadate solution.
The as-deposited film was the green and crystalline α-phase, which shows a p-type semiconductor behavior. After annealing at 250 °C, a reddish β-AgVO3 phase was obtained. The β-polymorph has a narrower band gap (2.12 eV) than the α-counterpart (2.45 eV), which was confirmed by theoretical band structure calculations. According to the team, the fact that these two polymorphic forms that only differ slightly have such different properties warrants further study.
- Electrodeposition of Silver Vanadate Films: A Tale of Two Polymorphs,
Abbas Vali, Hori P. Sarker, Hyung-Woo Jee, Attila Kormányos, Farinaz Firouzan, Noseung Myung, Ki-Jung Paeng, Muhammad N. Huda, Csaba Janáky, Krishnan Rajeshwar,
ChemPhysChem 2019.
https://doi.org/10.1002/cphc.201900558