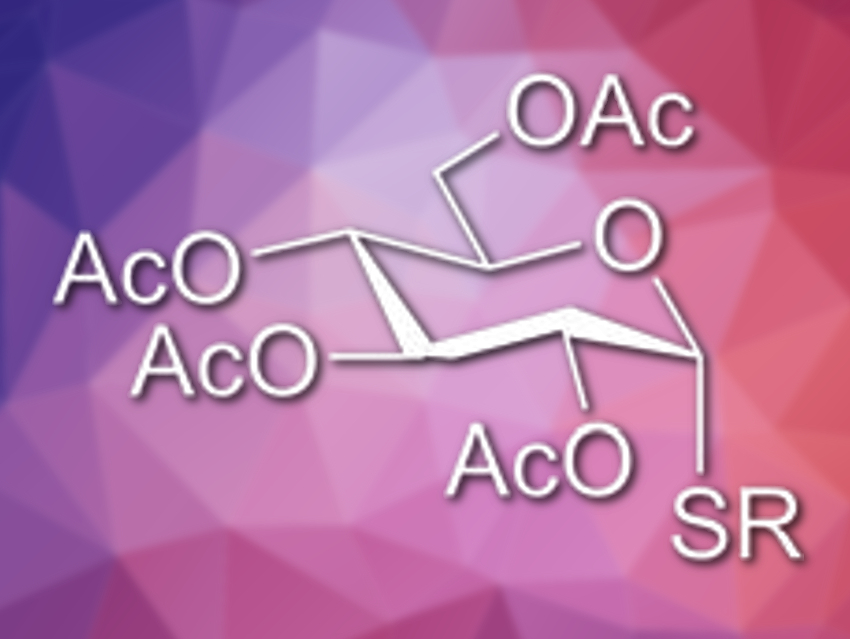
Thioglycosides are useful mimetics for natural carbohydrates. This is due to their stability to enzymatic hydrolysis and their close similarity to the natural O-glycosides. While many strategies exist for the synthesis of 1,2-trans-thio-linked glycomimetics, a general synthetic method for the analogous 1,2-cis-thio-linked glycosides had not yet been developed.
Anikó Borbás, University of Debrecen, Hungary, and colleagues have found that a radical-mediated thiol-ene coupling reaction of 1,2-unsaturated sugars, or glycals, can be used as a new stereoselective thioglycosylation method. Three types of acetylated glycals were reacted with a broad range of thiols under UV irradiation at temperatures between –120 °C and room temperature.
The team found that the reactions proceed with 1,2-cis-stereoselectivity and generally with high yield. Cooler temperatures were beneficial to the reaction. According to the researchers, these low-temperature thiol-ene coupling reactions provide a complementary strategy to existing methods for the synthesis of thioglycosides.
- Stereoselective Thioconjugation by Photoinduced Thiol-ene Coupling Reactions of Hexo‐ and Pentopyranosyl D– and L-Glycals at Low‐Temperature—Reactivity and Stereoselectivity Study ,
Viktor Kelemen, Miklós Bege, Dániel Eszenyi, Nóra Debreczeni, Attila Bényei, Tobias Stürzer, Pál Herczegh, Anikó Borbás,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201903095