Polycyclic aromatic hydrocarbons (PAHs) consisting only of fused aromatic rings are molecules with interesting structures. They occur as pollutants released by incomplete combustion processes. Due to their interesting optoelectronic properties, they can be used as materials for organic electronic devices (e.g., OLEDs). For further investigations, it is crucial to have reliable synthetic routes to these molecules.
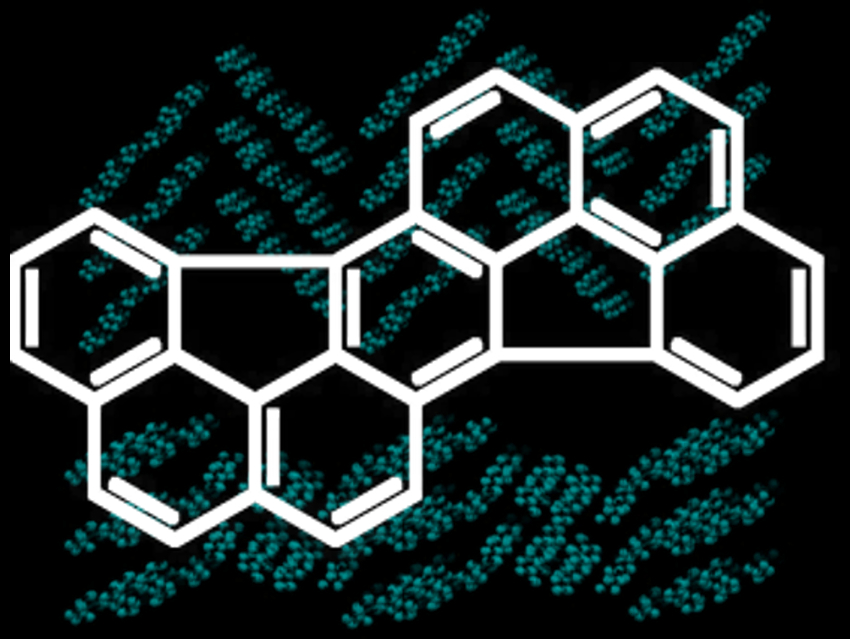
Hans-Joachim Knölker, Technische Universität Dresden, Germany, and colleagues have developed a synthetic path to the prominent PAH dibenzo[cde,opq]rubicene (pictured). The team’s eight-step synthesis relies on an iron-mediated [2+2+1] cycloaddition and a flash vacuum pyrolysis as key steps. A domino reaction consisting of Diels-Alder cycloaddition, CO extrusion, and aromatization has been used for the construction of the central benzene ring.
An X-ray crystal structure determination revealed two crystal modifications of dibenzo[cde,opq]rubicene with different packing modes (pictured in the background). According to the team, the synthesis should provide enough material for further studies, in particular, for environmental analytics. In addition, this synthetic strategy could pave the way for the synthesis of other PAHs.
- Synthesis and Crystal Structure of Dimorphic Dibenzo[cde,opq]rubicene,
Joghee R. Suresh, Glenn Whitener, Gabriele Theumer, Dirk J. Bröcher, Ingmar Bauer, Werner Massa, Hans‐Joachim Knölker,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201902915