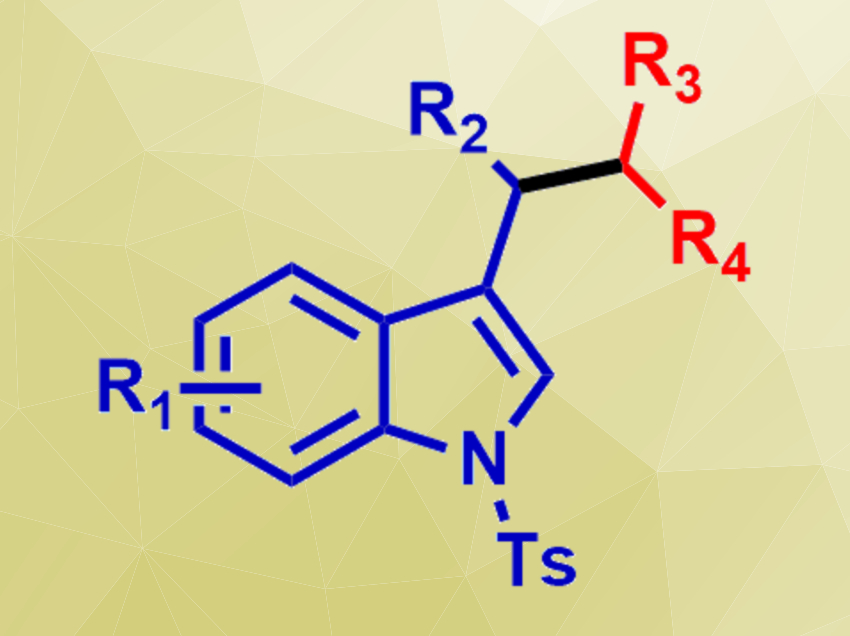
Indoles are widely used, e.g., in medicinal chemistry. Substitution at the 3-position is an important step in the synthesis of many complex indole derivatives. Therefore, efficient and environmentally benign protocols for this reaction are useful.
Umasish Jana, Jadavpur University, Kolkata, India, and colleagues have developed a strategy for the selective synthesis of structurally complex 3-substituted indoles (pictured) based on a tandem C–C bond formation and isomerization of 3-benzylidene-1-tosylindolines. The team used alcohols (R3R4HC–OH) as alkylating agents in the presence of inexpensive FeCl3 as a catalyst. The catalyst activates the alcohol, which is converted to a carbocation. This reactive intermediate can attack the substrate, form a new C–C bond, and cause the isomerization to the desired indole.
The reaction is environmentally friendly and convenient: It directly uses alcohols as alkylating agents, has a sustainable, non-toxic iron catalyst and mild reaction conditions, provides excellent yields of the products, and gives water as the only waste product.
- Iron-Catalyzed Functionalization of 3-Benzylideneindoline Through Tandem Csp2–Csp3 Bond Formation/Isomerization with π-Activated Alcohols,
Rupsa Chanda, Baitan Chakraborty, Gopal Rana, Umasish Jana,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901181