A cursory glance at the organic chemistry literature might lead you to conclude that by this point, all the simple motifs into which a bunch of carbon and hydrogen atoms might be arranged will have been synthesized. You might think the triangles, squares, boats, and chairs are all done. But even some seemingly simple constructions have not been prepared at the laboratory bench so far.
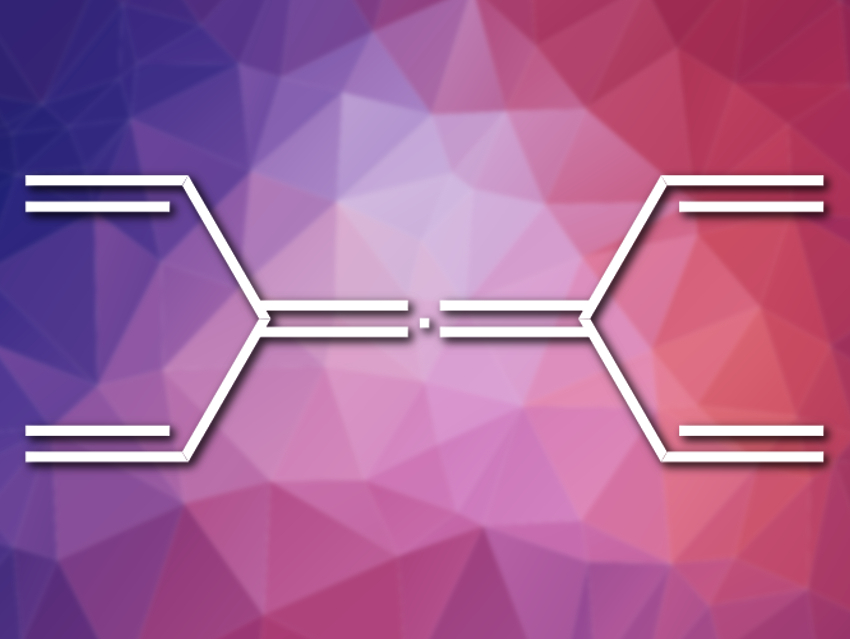
Take tetravinylallene (pictured), for instance. We can give it the name (3,5-divinylhepta-1,3,4,6-tetraene), which might make it sound more complicated than it is to a chemical nomenclature novice, but either way, it is composed of a single sp-hybridized carbon and ten sp2-hybridized carbons. Surely, the novice might suggest, that has been synthesized already.
Molecular Whimsy
Well, this structure may well have been talked about for decades, perhaps sketched in a lab book, or pieced together by a student from plastic ball-and-stick components to make a whimsical molecular model. But, at long last, tetravinylallene has been constructed from simpler starting materials by Michael S. Sherburn and colleagues, Australian National University, Canberra. The researchers point out that the simpler chemical cousin of their target, 1,3-divinylallene, was reported in 1975 [1], and the far less stable relative 1,1-divinylallene was not made until 2011 [2]. Now, the team has found a route to tetravinylallene.
The team used a multi-step procedure in which the final, key step was a process involving a palladium-catalyzed, Negishi-type cross-coupling reaction. This encompassed a 1,5-transposition of a penta-2-en-4-yn-1-ol methanesulfonate to give the final, surprisingly stable product. The material was isolated as a colorless oil following purification by flash chromatography. The product needs to be stored in a benzene solution, because storage as a neat liquid causes decomposition to an insoluble, probably polymeric white solid.
Generic Bonds and Building Blocks
Of course, this simple generic synthesis opens up the possibility of modifying and functionalizing this fundamental hydrocarbon unit. The team asserts that their approach “breaks new ground in swift structural complexity creation.” Aside from the aesthetic appeal of this target, the researchers were quick to perceive the molecule as an entry point for σ-bond-forming reactions.
The team went on to produce several substituted versions of the molecule with the aim of understanding how substituents and substitution order might affect stability. Some of those products were shorter-lived than others—one of them had a half-life a thirtieth of that of its parent structure. Nevertheless, these substituted structures can undergo subsequent ring closures to generate much greater complexity than is perceived in the initial fundamental building block of tetravinylallene.
- Tetravinylallene,
Cecile Elgindy, Jas S. Ward, Michael S. Sherburn,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201908496
References
- [1] Synthesis of 1,3,4,6-Heptatetraene (Divinylallene),
Ursula Mödlhammer, Henning Hopf,
Angew. Chem. Int. Ed. Engl. 1975, 14, 501–502.
https://doi.org/10.1002/anie.197505011 - [2] 1,1-Divinylallene,
Katie M. Cergol, Christopher G. Newton, Andrew L. Lawrence, Anthony C. Willis, Michael N. Paddon-Row, Michael S. Sherburn,
Angew. Chem. Int. Ed. 2011, 50, 10425–10428.
https://doi.org/10.1002/anie.201105541