Fluorinated compounds are widely used in industry, especially in materials, agrochemicals, and pharmaceuticals. The C–F bond activation of polyfluorinated compounds is a commonly used method to obtain industrially valuable derivatives. Various catalysts such as transition-metal complexes or Lewis-acidic species have been used to achieve such transformations. For example, aluminum chlorofluoride (ACF), AlClxF3-x (x ≈ 0.05–0.3)—an amorphous, nanoscopic, solid Lewis acid—proved to be a suitable catalyst for heterogeneous catalytic reactions.
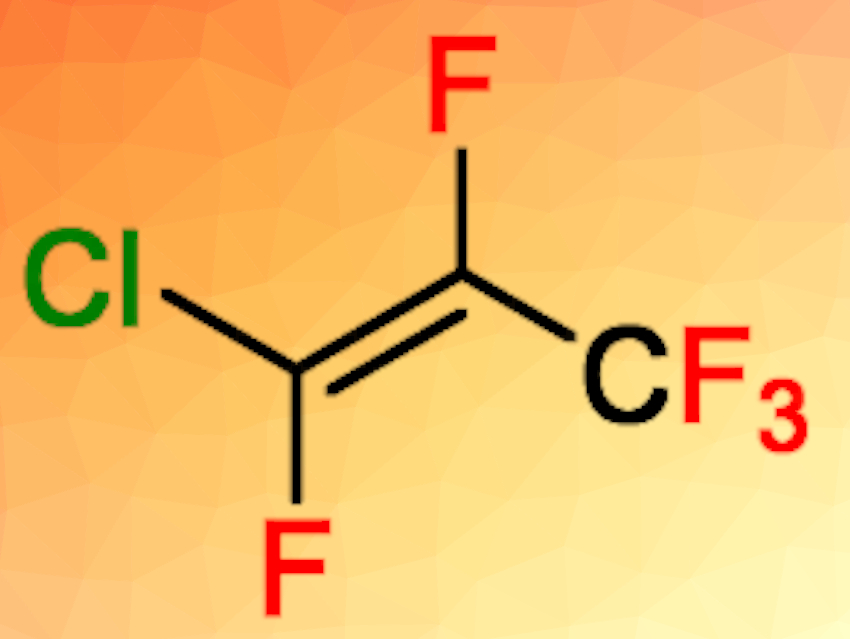
Thomas Braun, Humboldt University Berlin, Germany, and colleagues have developed unprecedented catalytic fluorine/chlorine exchange reactions at fluoromethanes and fluoroalkenes to give chlorinated methanes or fluorochloroalkenes (examples pictured below). The reactions take place using ACF as the catalyst in the presence of chlorinated silanes or germanes. Monofluoromethane, for example, was reacted at 70 °C in C6D12 and converted to monochloromethane in a yield of 72 %.

Magic angle spinning (MAS) NMR surface studies provided information on the interaction of the reactants with the ACF surface. The results suggest the presence of silylium-like species, which might play a role in the reaction mechanism. The developed ACF/ClSiEt3 system can provide a useful tool for chlorodefluorinations of both fluoroalkyl and fluoroalkenyl compounds.
- Chlorodefluorination of Fluoromethanes and Fluoroolefins at a Lewis Acidic Aluminum Fluoride,
Xinzi Pan, Maria Talavera, Gudrun Scholz, Thomas Braun,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200029