Molecules with thermally activated delayed fluorescence (TADF) can be used in, e.g., organic light-emitting diodes (OLEDs). Blue and green TADF OLEDs have achieved high external quantum efficiencies (EQEs). However, red or near-infrared (NIR) TADF emitters have lagged behind the green and blue ones due to their narrow energy gaps. These often lead to substantial energy loss through non-radiative decay.
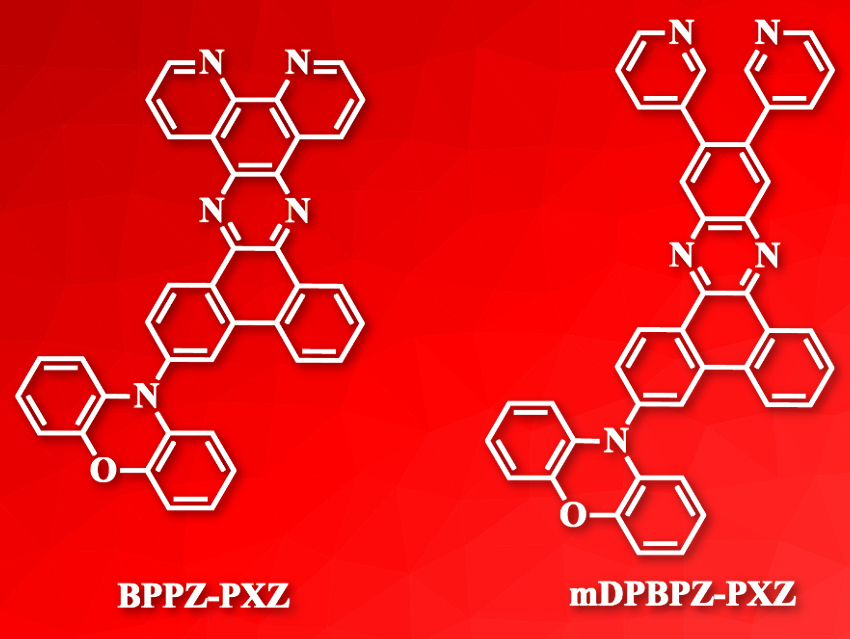
Xiao-Hong Zhang, Soochow University, Suzhou, Jiangsu, P. R. China, Chun-Sing Lee, City University of Hong Kong, Hong Kong SAR, and colleagues have developed two efficient red TADF compounds, BPPZ‐PXZ and mDPBPZ‐PXZ (pictured). The molecules combine rigid planar phenoxazine segments (pictured on the bottom) with large heterocyclic aromatic hydrocarbon segments. Both compounds were synthesized via a 3‐(10H‐phenoxazin‐10‐yl)phenanthrene‐9,10‐dione intermediate, which was obtained from 3‐bromophenanthrene‐9,10‐dione and phenoxazine via a Buchwald–Hartwig cross‐coupling reaction.
Both TADF molecules obtained near 100 % photoluminescence quantum yields in films doped with 4,4′‐di(9H‐carbazol‐9‐yl)‐1,1′‐biphenyl (CBP). OLEDs based on BPPZ‐PXZ achieved EQEs of 25.2 %—the highest efficiency among reported red TADF OLEDs.
- Red/Near-Infrared Thermally Activated Delayed Fluorescence OLEDs with Near 100 % Internal Quantum Efficiency,
Jia-Xiong Chen, Wen-Wen Tao, Wen-Cheng Chen, Ya-Fang Xiao, Kai Wang, Chen Cao, Jia Yu, Shengliang Li, Feng-Xia Geng, Chihaya Adachi, Chun-Sing Lee, Xiao-Hong Zhang,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201906575
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

