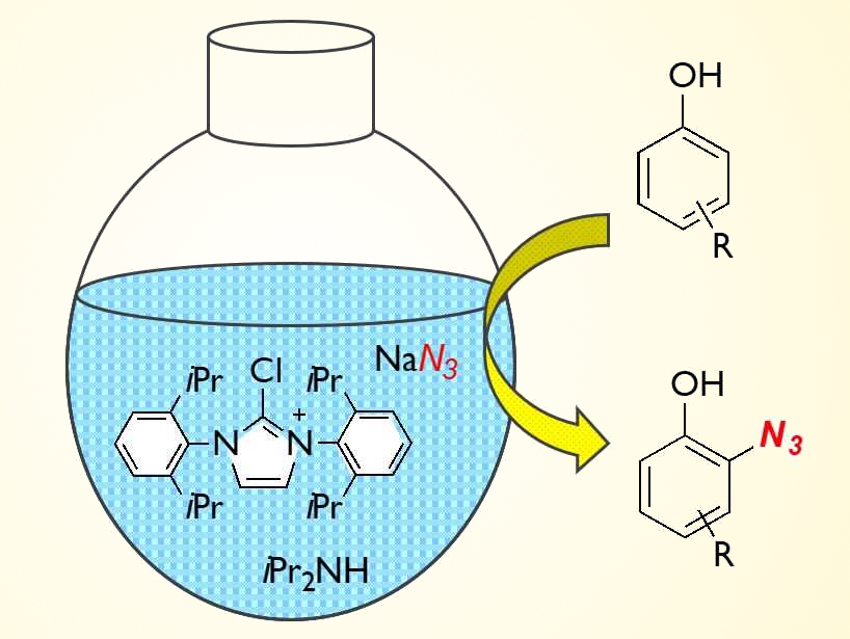
Aryl azides have become increasingly important in biological chemistry, for example, in 1,3‐dipole reactions of aryl azides and alkynes, which are useful in drug discovery. There are various synthetic methods to obtain aryl azides from aryl amines and aryl halides. However, direct azidation reactions of arenes had not been developed so far.
Mitsuru Kitamura and colleagues, Kyushu Institute of Technology, Kitakyushu, Japan, have developed a method for the direct azidation of phenols. By reacting a chloroimidazolium chloride with bulky aryl substituents (pictured), sodium azide, and phenol in the presence of a secondary amine, an ortho-azidation of phenols was achieved. The reaction proceeds via an azidoimidazolinium salt that transfers the azide group to the phenol.
In this reaction, the choice of solvent is important. When an aprotic polar solvent such as acetonitrile is used, the yield of the desired azidophenols is low and an N3-bridged byproduct is formed from the imidazolium salt. However, this byproduct formation is suppressed when using a protic solvent. Optimal results were achieved when methoxyethanol was used.
- Direct Azidation of Phenols,
Mitsuru Kitamura, Kento Murakami, Tatsuya Koga, Takashi Eto, Akihiro Ishikawa, Hirokazu Shimooka, Tatsuo Okauchi,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900967