Magnesium borohydride, Mg(BH4)2, is one of the few hydrogen storage materials which could meet all the performance requirements for practical, on-board fuel-cell applications. However, overcoming the extremely slow kinetics of reversible hydrogen release is challenging.
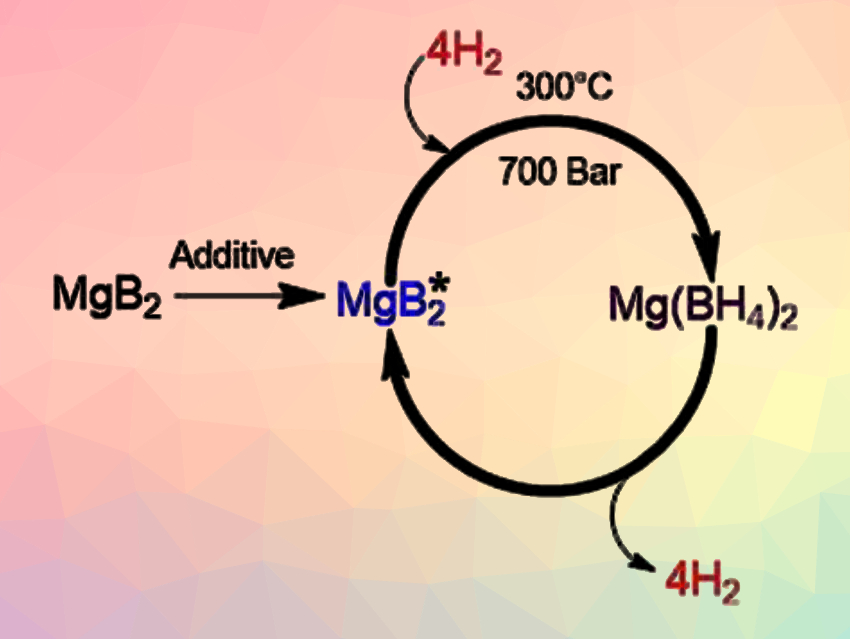
Godwin Severa, Craig Jensen, University of Hawaii at Manoa, Honolulu, USA, and colleagues have developed a magnesium-boride-based material that is highly activated towards hydrogenation. The material could enable efficient hydrogen cycling of magnesium boride (MgB2) to Mg(BH4)2 under fuel-cell-relevant conditions. The hydrogenation of MgB2 to Mg(BH4)2 has previously been achieved only at the forcing conditions of 900 bar and 400 °C.
The team showed that mechanical milling of MgB2 with additives such as tetrahydrofuran (THF), magnesium, and/or magnesium hydride results in a significant improvement of the kinetics of its bulk hydrogenation. This allows the desired transformation to be accomplished at 300 °C under 700 bar H2—the mildest conditions reported so far for this reaction—while achieving 54–71 % conversion to Mg(BH4)2. The team suggests that milling with the additives could introduce defects in the MgB2 structure which enhance hydrogenation.
- Kinetic Enhancement of Direct Hydrogenation of MgB2 to Mg(BH4)2 upon Mechanical Milling with THF, MgH2, and/or Mg,
Cody Sugai, Stephen Kim, Godwin Severa, James L. White, Noemi Leick, Madison B. Martinez, Thomas Gennett, Vitalie Stavila, Craig Jensen,
ChemPhysChem 2019, 20, 1301–1304.
https://doi.org/10.1002/cphc.201801187