Battery-supercapacitor hybrids (BSH) combine electrodes from both technologies. They could provide the best of both systems: the capacity advantage of batteries and the rate performance of supercapacitors. However, this hybrid technology is still very limited in terms of energy density. This limitation is mainly due to the side reactions at the supercapacitor electrode.
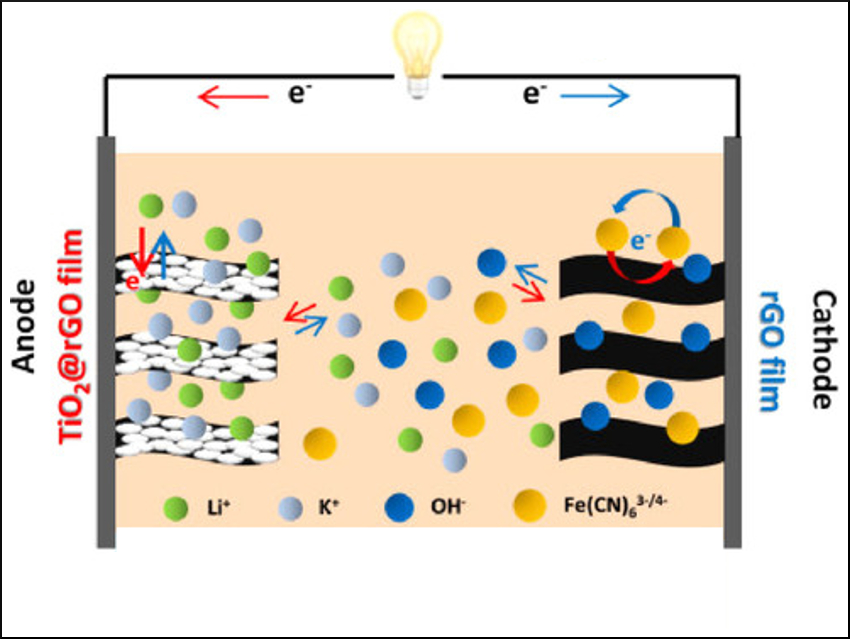
Xiaoli Zhao, Xiaowei Yang, and colleagues, Tongji University, Shanghai, China, have used what they call a “potential fringe” strategy to increase the energy density of a BSH. They constructed an alkaline BSH (pictured) using reduced graphene oxide (rGO) hydrogel films as the cathode and TiO2@rGO hydrogel films as the anode, with LiOH/K4Fe(CN)6 as electrolytes.
in this BSH, two fast redox reactions (“potential-fringe reactions”) occur just below and just above the onset potentials of hydrogen and oxygen evolution, respectively. In this way, the team kinetically suppressed water decomposition and increased the stability window of the electrolyte. This led to a higher Coulombic efficiency and energy density.
This approach had already been used for supercapacitors. This work demonstrates that it works just as well for hybrid systems. BSHs are still at an early stage, but once high energy and power densities can be achieved simultaneously, they should be able to power portable electronic devices.
- Integrating Fast Potential‐Fringe Battery Reactions for High‐Voltage Battery‐Supercapacitor Hybrid Energy Storage Systems,
Ziwei Xiao, Shengping Wang, Xiaojun Yan, Congcong Liu, Xiaoli Zhao, Xiaowei Yang,
Batteries & Supercaps 2019.
https://doi.org/10.1002/batt.201900070