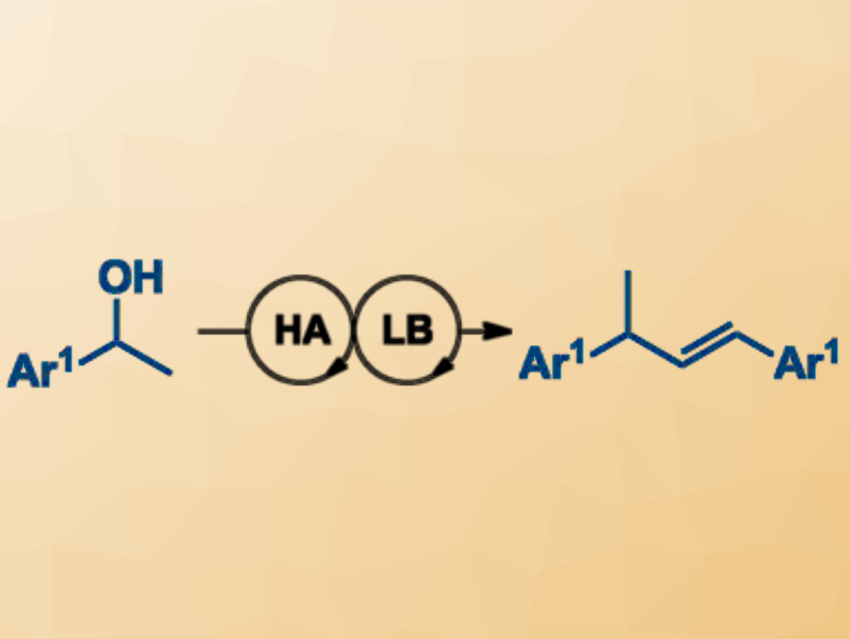
The direct functionalization of alcohols is a useful method for the formation of new carbon–carbon bonds. The starting materials are readily available and water is formed as the only by-product. Catalytic reactions of this type are particularly interesting. Brønsted acids, for example, can act as catalysts in the dehydrative homocoupling of benzylic alcohols and related couplings with nucleophiles.
Marlene Böldl and Ivana Fleischer, University of Tübingen, Germany, have found that a catalyst combination of toluenesulfonic acid as a Brønsted acid with the Lewis base triphenylphosphine leads to improved yields and shorter reaction times in the homocoupling of alcohols (pictured).
A variety of olefins were synthesized from benzylic alcohols under relatively mild conditions, i.e., in 1,2‐dichloroethane (DCE) at 60 °C within 18 h in air. Additionally, the method can be used for the dehydrative hydroarylation of benzylic alcohols with electron‐rich arenes, using only the Brønsted acid under otherwise identical reaction conditions.
To understand the role of the Lewis base, the team performed a series of mechanistic investigations, including the synthesis of plausible reaction intermediates as well as reaction progress and NMR studies. The results indicate that the phosphine stabilizes cationic reaction intermediates and prevents oligomerization side reactions.
- Dehydrative Coupling of Benzylic Alcohols Catalyzed by Brønsted Acid/Lewis Base,
Marlene Böldl, Ivana Fleischer,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900965