Peptoids. i.e., oligomers of N-substituted glycine, are peptidomimetic molecules. Like proteins, they can fold into specific secondary structures guided by their sequence. However, using a peptoid’s structure to control its function can be challenging.
Galia Maayan and colleagues, Technion–Israel Institute of Technology, Haifa, have investigated one such relationship between structure and function: They found a direct correlation between the structure of short peptoid ligands and the photoluminescence of RuII ions coordinated by these ligands.
The team attached bipyridine (bipy) groups to a variety of peptoid oligomers, differing in sequence and structure. These peptoids were used to form photoluminescent ruthenium metal complexes (with three ligands per metal). The secondary structure of each peptoid-complex was characterized by circular dichroism (CD) spectroscopy and its photoluminescence was measured.
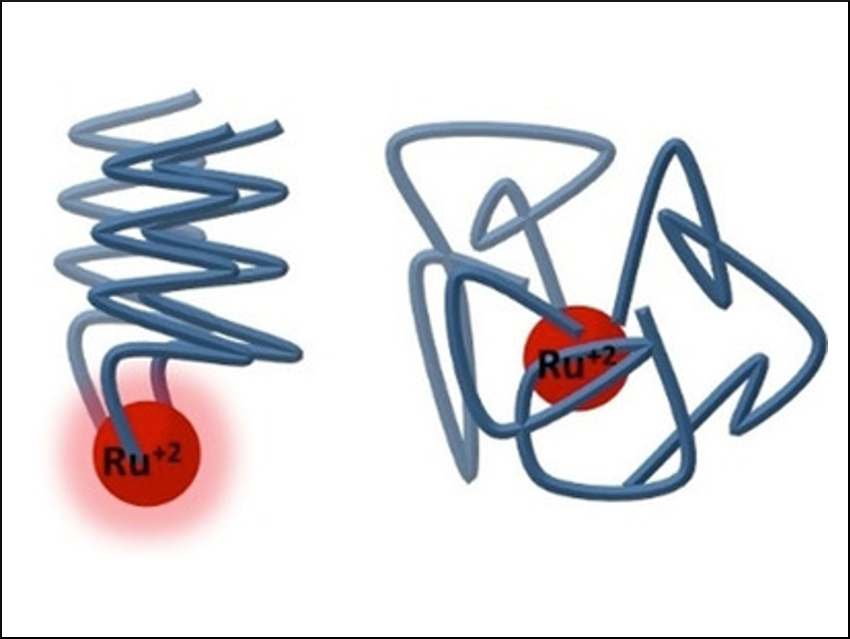
The researchers found that the photoluminescence of the bipy-ruthenium complexes embedded within helical peptoids (pictured left) is retained. In contrast, the emission of non-helical ruthenium peptoid complexes (pictured right) is quenched. It is possible to fine-tune the luminescence of the complex by modulating the structure of the peptoid, i.e., by altering its monomer sequence. This unique structure-function correlation has potential applications in sensing and photocatalysis.

- Sequence and Structure of Peptoid Oligomers Can Tune the Photoluminescence of an Embedded Ruthenium Dye,
Lieby Zborovsky, Hagar Tigger-Zaborov, Galia Maayan,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201901494