Cephalosporin Antibiotics
Antibiotics save countless human lives—modern medicine without them is unimaginable. The largest proportion by volume of industrially produced antibiotics today are cephalosporins, structural variants of the first antibiotic, penicillin. Unfortunately, their production generates a considerable amount of waste products. Harald Gröger, Bielefeld University, Germany, and colleagues have shown that a newly developed, more ecological synthetic route is suitable for the production of a wide variety of cephalosporin antibiotics.
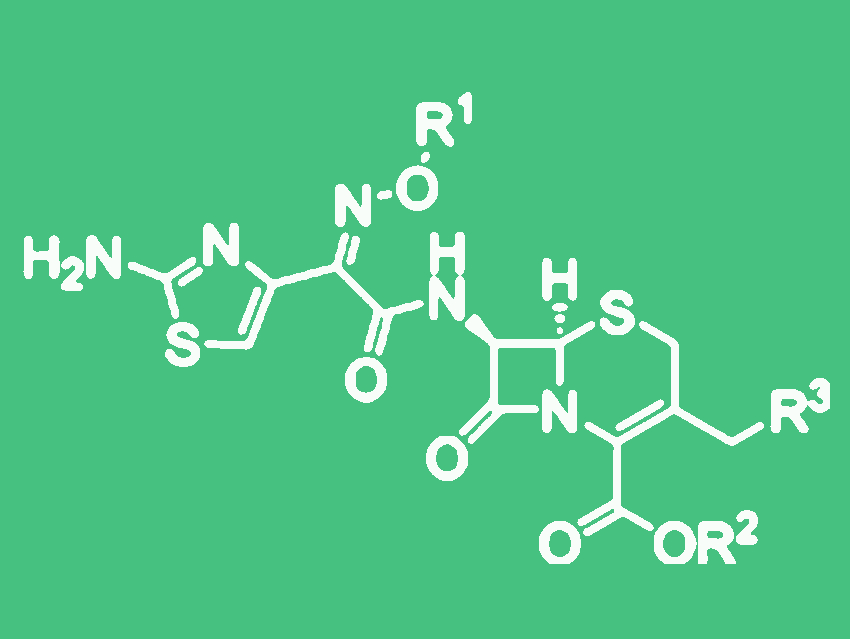
Penicillin is among the ß-lactam antibiotics, a class of substances whose common component is a lactam. Cephalosporins, the second most significant subclass of the ß-lactam antibiotics, contain a bicyclic system made of the ß-lactam ring and a six-membered ring made of sulfur, nitrogen, and carbon atoms. The various therapeutically useful drugs in this class differ in their side chains, which can be attached at various places on the basic framework.
The production of cephalosporin antibiotics is a semi-synthetic process. A fermentation first yields cephalosporin C, which is then enzymatically split to form 7-aminocephalosporanic acid (7-ACA). Different drugs are then produced from 7-ACA through chemical syntheses. Production of third-generation cephalosporin antibiotics involves the attachment of an amino group of the lactam ring to a building block based on (Z)-(2-aminothiazol-4-yl)methoxyiminoacetic acid. Both the production of this reactant and the binding reaction are ecologically unfavorable because they result in large amounts of partially problematic waste products. These are formed because a variety of reagents are needed for activation, for the protection of groups that are not supposed to react, and for the coupling itself, depending on which drug is being produced.
Improved Atom Economics
The team recently cooperated with the Provadis School of International Management and Technology and the generic drugs manufacturer Sandoz GmbH, Kundl, Austria, to develop an interesting alternative for this type of amidation reaction, and used it to make cefotaxime. The key to their success was the use of tosyl chloride, an established, inexpensive coupling reagent, in combination with methanol as an unproblematic solvent. The only side product is toluene sulfonyl acid, which is more attractive from the toxicological point of view since it requires neither protective groups nor activators that could form waste products. “This is a very favorable process with regard to atom economics,” says Gröger. Atom economics considers the percentage of atoms in the starting materials that are actually transferred to the products in a chemical reaction.
The researchers have been able to demonstrate that their amidation method is generally suitable for the production of third-generation cephalosporin antibiotics. In a research project funded by the Deutsche Bundesstiftung Umwelt (DBU, German Foundation for the Environment), they successfully synthesized three further cephalosporin antibiotics: cefpodoxime, cefpodoxime proxetil, and a precursor of ceftazidime. Despite non-optimized reaction conditions, their yields of 82–95 % are very high. “It is particularly noteworthy that many different functional groups at diverse positions on the 7-ACA-based starting molecules are tolerated,” says Gröger. “Our ecologically and economically advantageous synthetic route offers the prospect of broad application in the industrial production of cephalosporin antibiotics.”
- General Synthesis of Industrial Cephalosporin-Based Antibiotics Through Amidation with Tosyl Chloride as a Coupling Reagent,
Matthias Pieper, Herbert Schleich, Harald Gröger,
Eur. J. Org. Chem. 2019, 2019, 3259–3263.
https://doi.org/10.1002/ejoc.201900277