The enzyme pyruvate formate-lyase (PFL) plays a key role in the anaerobic glucose metabolism of many microbes. (PFL) catalyzes the reversible conversion of pyruvate and coenzyme A (CoA) into formate and acetyl‐CoA in two half‐reactions. For the second half‐reaction to take place, the S−H group of CoA must enter the active site of the enzyme to retrieve a protein‐bound acetyl group. However, CoA is bound at the protein surface and the active site is buried in the protein’s interior, about 20–30 Å away. The mechanism of CoA entry has remained unclear so far.
To shed light on this mechanism, David M. Smith, Ruđer Bošković Institute, Zagreb, Croatia, and colleagues have performed extensive molecular dynamics (MD) simulations on both acetylated (Ac) and non-acetylated model systems of PFL. These models represent PFL before and after the first half‐reaction and were used to examine the possible effect of enzyme acetylation.
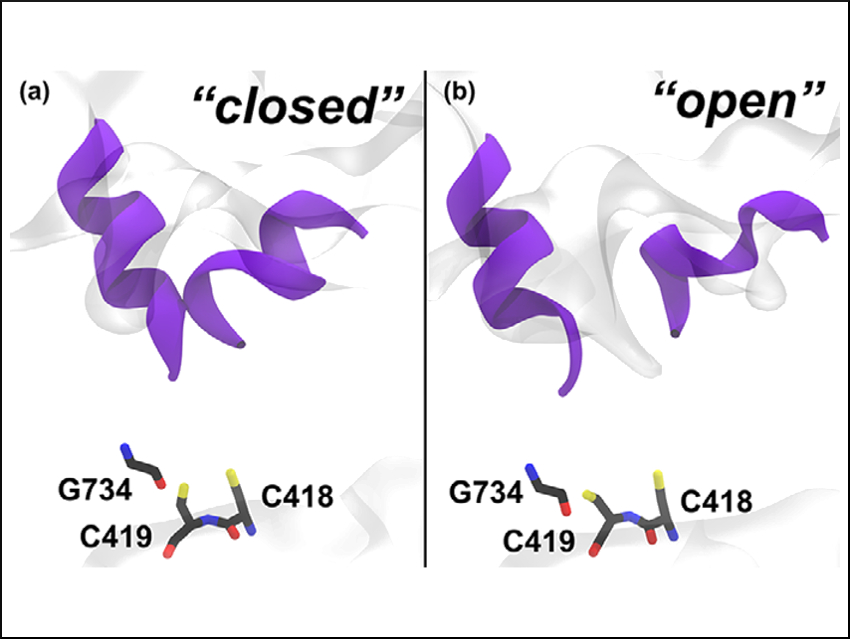
The team showed that the acetylated forms of PFL have an enhanced flexibility in the active site. This results in a series of subtle structural rearrangements, which increase the probability of finding the CoA entry channel in an “open” state (pictured right). This result suggests that the acetylation of the enzyme has an important functional role: The formation of the acyl intermediate initiates a subtle signaling cascade that influences the protein dynamics and facilitates the entry of the second substrate.
- The Influence of Chemical Change on Protein Dynamics: A Case Study with Pyruvate Formate-Lyase,
Marko Hanževački, Karmen Čondić‐Jurkić, Radha Dilip Banhatti, Ana‐Sunčana Smith, David M. Smith,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201900663