One goal of catalysis is to maximize the atom- and energy-efficiency of chemical processes. In contrast to bulk materials, finely dispersed metals on support materials allow researchers to tailor the catalytic site with atomic precision. Addition or removal of a single atom can alter the structure or electronic properties of the active site and, thereby, have a decisive impact on catalysis.
Javier Pérez-Ramírez, Sharon Mitchell, ETH Zurich, Switzerland, and colleagues have stabilized low-nuclearity palladium clusters on exfoliated carbon nitride (ECN). Monomeric, dimeric, and trimeric palladium complexes were then deposited from solutions of palladium compounds onto the support. This led to three catalytic materials—Pd1/ECN, Pd2/ECN, and Pd3/ECN—each with metal loadings of approximately 0.5 wt%.
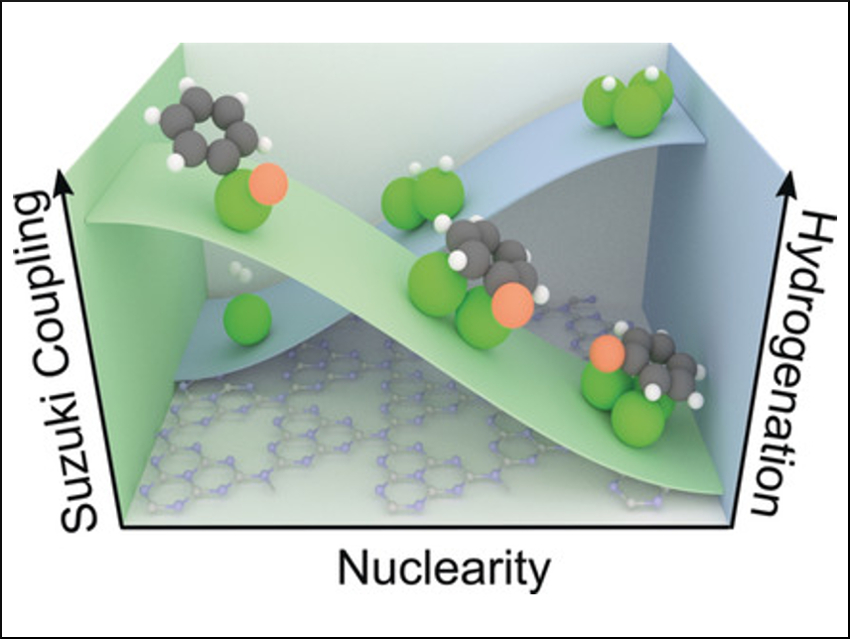
The researchers then explored the correlation between nuclearity and activity in two different reactions: the semi-hydrogenation of alkynes and Suzuki coupling reactions. The team found that Pd3/ECN was more active in the selective hydrogenation of various functionalized alkynes as a result of a lower hydrogen activation barrier. In contrast, Pd1/ECN excelled at Suzuki coupling with distinct chemoselectivity and greater stability. The researchers believe the findings provide practical guidance for the design of heterogeneous catalysts with a defined number of metal atoms.
- Atom-by-Atom Resolution of Structure-Function Relations over Low-Nuclearity Metal Catalysts,
Evgeniya Vorobyeva, Edvin Fako, Zupeng Chen, Sean M. Collins, Duncan Johnstone, Paul A. Midgley, Roland Hauert, Olga V. Safonova, Gianvito Vilé, Núria López, Sharon Mitchell, Javier Pérez-Ramírez,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201902136Javier Javier Pérez-Ramírez is a recipient of the 2019 Paul H. Emmett Award in Fundamental Catalysis for the design of innovative catalytic processes that address energy, resource, and environmental challenges. Read about the award in Angewandte Chemie and in an interview published in ChemCatChem.