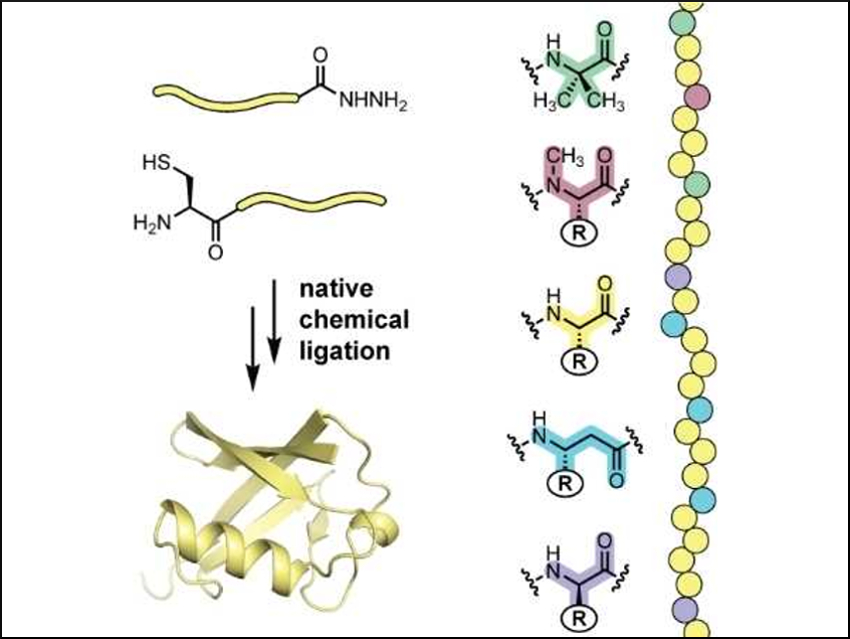
Designing molecules with protein-like functions is a challenge. Such protein mimetics have potential biomedical applications and can be used to study natural systems. One tactic toward protein mimicry is to create artificial backbones that fold in defined ways. A key challenge has been to increase the functional complexity possible in such entities.
W. Seth Horne and colleagues, University of Pittsburgh, PA, USA, have developed artificial-backbone mimics of ubiquitin, a multifunctional signaling protein. The natural sequence of ubiquitin’s backbone connectivity was systematically changed. These ubiquitin mimics were prepared using native chemical ligation (NCL). NCL is a chemical method for the preparation of polypeptides or proteins by connecting unprotected peptide segments.
The team found that some of the ubiquitin mimics are recognized by enzymes that use natural ubiquitin to modify other proteins in the cell. In addition, proteins tagged with the ubiquitin mimics can be recognized by other proteins for downstream pathways. The team hopes these results will broaden the scope of protein mimicry possible in artificial backbones and enable their application to previously inaccessible systems.
- Exploring the Functional Consequences of Protein Backbone Alteration in Ubiquitin through Native Chemical Ligation,
Halina M. Werner, Samuel K. Estabrooks, G. Michael Preston, Jeffrey L. Brodsky, William Seth Horne,
ChemBioChem 2019.
https://doi.org/10.1002/cbic.201900225