Nanostructured materials have a broad range of applications. Organic π-conjugated molecules, for example, have tunable optoelectronic properties and could be useful for assembling functional nanomaterials. It can, however, be challenging to control the ordered assembly from molecules to nanostructured materials and, thus, enhance the material’s performance. Noncovalent interactions between individual molecules are known to play a key role in governing self-assembly. Solvent effects may also influence aggregation and should be taken into account.
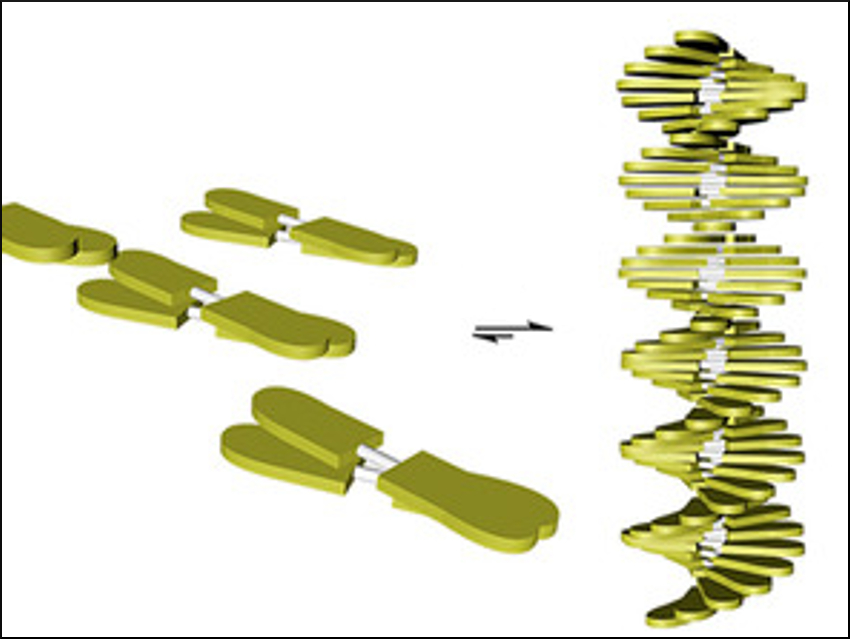
David González-Rodríguez and colleagues, Universidad Autónoma de Madrid, Spain, have prepared two π-conjugated, diacetylene-linked oligo(phenylenevinylene) molecules that form different nanostructures in different solvents (pictured below). The diacetylene‐bridged compounds were produced by a direct Glaser homocoupling of ethynyl‐substituted precursors in the presence of Cu2+.
The team studied the nanostructures using optical spectroscopy and atomic force microscopy (AFM) measurements. The solubility of the molecules and their corresponding assemblies in water or in organic solvents is controlled by the peripheral substituents attached at the oligomer edges, either tetraethylene glycol tails (pictured in blue) or dodecyl chains (pictured in yellow).
In water (pictured in blue), the rod-like molecules self-assemble into robust, uniform micellar structures. In contrast, a fibrillar morphology is obtained in alkanes (pictured in yellow) in a cooperative nucleation-elongation process influenced by the low rotation barrier of the diacetylene bond in the linker. This shows that the supramolecular assembly is strongly dependent on the nature of the solvent.

- Self-Assembly of Diacetylene-Bridged Phenylenevinylene Oligomers in Water and Organic Solvents,
Miguel García‐Iglesias, María José Mayoral, David Serrano‐Molina, Fátima Aparicio, Violeta Vázquez‐González, David González‐Rodríguez
ChemPlusChem 2019, 84, 488–492.
https://doi.org/10.1002/cplu.201900207