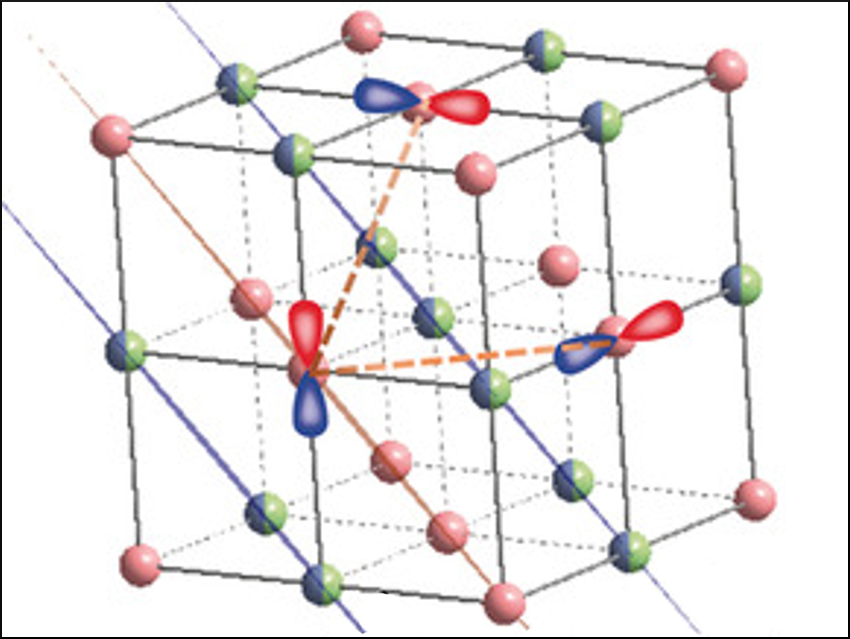
Lithium-ion batteries possess a high energy density. Reversible lattice-oxygen redox (l-OR) chemistry stabilizes the crystal structure of lithium-rich cathodes and gives them a high capacity. However, l-OR chemistry remains poorly understood.
Xiqian Yu, Lin Gu, Fangwei Wang, Chinese Academy of Sciences, Beijing, China, Wanli Yang, Lawrence Berkeley National Laboratory, CA, USA, and colleagues have studied the influence of structural disorder on l-OR chemistry. The team used a lithium-rich oxide with a 3D-disordered cation framework, Li1.2Ti0.35Ni0.35N0.1O1.8F0.2, as a model.
Neutron powder diffraction and atomic-resolution scanning transmission electron microscopy (STEM) revealed that the material has a stable oxygen lattice structure during the l-OR process. This result contrasts with the distorted oxygen lattice of traditional lithium-rich layered oxides and is a consequence of the disordered cations.
According to the researchers, an optimized structure promotes reversible l-OR chemistry. It could improve the reversible capacity of high-energy-density lithium-rich oxide cathodes.
- Stabilizing the Oxygen Lattice and Reversible Oxygen Redox Chemistry through Structural Dimensionality in Lithium-Rich Cathode Oxides,
Enyue Zhao, Qinghao Li, Fanqi Meng, Jue Liu, Junyang Wang, Lunhua He, Zheng Jiang, Qinghua Zhang, Xiqian Yu, Lin Gu, Wanli Yang, Hong Li, Fangwei Wang, Xuejie Huang,
Angew. Chem. Int. Ed. 2019, 58, 4323–4327.
https://doi.org/10.1002/anie.201900444