Highly Resorptive Metal–Organic Frameworks
Gases and pollutants can be filtered from air and liquids using porous, crystalline materials, such as metal–organic frameworks (MOFs). To further partition the pores of such materials and enhance their sorption capacity, Xianhui Bu, California State University Long Beach, Long Beach, USA, Pingyun Feng, University of California, Riverside, USA, and colleagues have developed a fast and versatile two-in-one synthetic strategy, combining metal coordination with the covalent chemistry of light elements. The new pore-space-partitioned material could be used as a highly efficient adsorbent of ammonia.
The structure of MOFs is a coordinative network of metals with organic linkers, which builds up a large and symmetric three-dimensional porous network. Gases can diffuse in and out of the pores. Once in a MOF, gas molecules adsorb at adsorption sites provided by the metal ions and the linker molecules. However, small gas molecules such as CO2, acetylene, and ammonia do not need large pores to be trapped, and it turns out that sometimes a denser network and more adsorption sites can enhance the capacity of a MOF.
Pore-Space Partitioning
Therefore, the team attempted to partition the pores with covalent ligands—spacer molecules that assemble through chemical reactions. Partitioning has the additional advantage that it could make the MOF more stable. Instability is one of the reasons why MOFs have not found widespread use yet, although they are far more efficient gas sorption materials than, for example, zeolites and activated carbon.
The researchers chose the aromatic molecule pyridine-4-boronic acid as a partitioning molecule. This is an unusual ligand. It combines two different light elements with complementary reactivity: boron is a Lewis acid and tends to catch agents with high electron density, while the pyridinic nitrogen is a Lewis base searching for Lewis acids to react with. Under normal conditions, these molecules would simply attack each other and cause many non-targeted reactions.
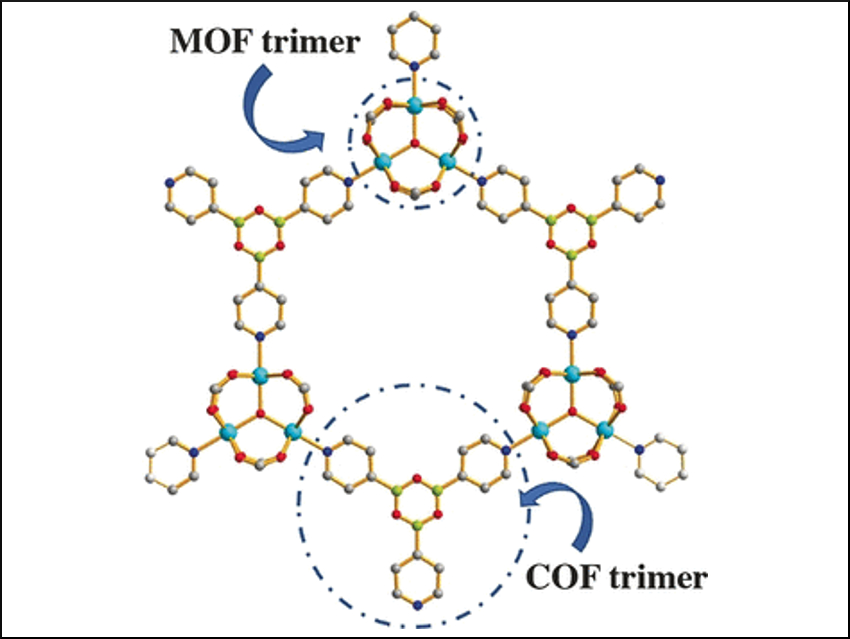
However, this did not happen here because the team integrated the pyridine-4-boronic acid reaction into the metal coordination reaction that builds up the MOF. Both covalent and coordinative reactions acted synergistically and protected the pyridine-4-boronic acid from side reactions. A trimer formed that fitted neatly into the hexagonal pores of the MOF. The result was a MOF with an integrated covalent organic network, or “pore-space partitioned MOF”, providing many new sites for gas adsorption.
The scientists synthesized several of these MOFs, each with a different combination of metals and organic ligands. The new pore-space-partitioned MOFs showed better gas uptakes than those that were unpartitioned. Moreover, the exposed boron Lewis acid sites of the partitioning ligands permitted ammonia uptake with a high packing density. This work presents an advancement in MOF synthesis and performance. Reactions that were not deemed possible—such as neat trimerization of a pyridine boronic acid—were achieved and may lead to highly useful components.
- A Tale of Two Trimers from Two Different Worlds: A COF-Inspired Synthetic Strategy for Pore-Space Partitioning of MOFs,
Yanxiang Wang, Xiang Zhao, Huajun Yang, Xianhui Bu, Yong Wang, Xiaoxia Jia, Jinping Li, Pingyun Feng,
Angew. Chem. Int. Ed. 2019, 58, 6316–6320.
https://doi.org/10.1002/anie.201901343

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

