The Chichibabin reaction, an amination first discovered in 1914 [1], can install a primary amino group (–NH2) at the C2 position of pyridine derivatives using, e.g., sodium amide. However, the reaction usually needs harsh reaction conditions. In addition, only a few successful examples of the reaction using primary alkyl amines have been reported. This is probably due to the inaccessibility of the corresponding alkyl metal amides.
Shunsuke Chiba, Nanyang Technological University, Singapore, and colleagues have developed a concise protocol for the Chichibabin amination that allows efficient access to a range of 2-aminopyridines and their derivatives under mild reaction conditions. The team used sodium hydride (NaH) as a base in the presence of lithium iodide (LiI) and primary amines in tetrahydrofuran (THF) at reaction temperatures ranging from 65 °C to 85 °C. The reaction introduces the desired primary alkyl amines at the C2 position in good to excellent yields.
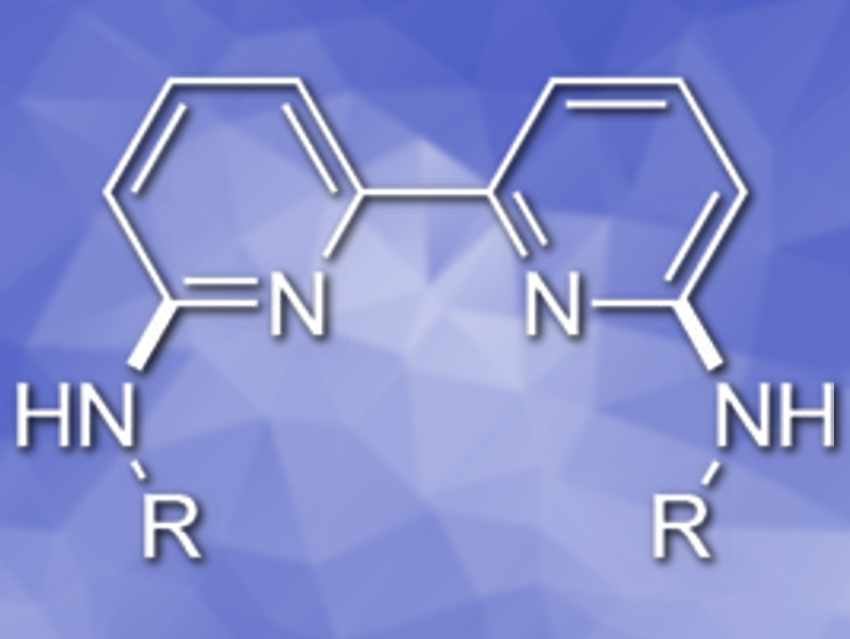
The method can be scaled up to a 20-mmol-scale. It can also be extended to a double amination of 2,2′-bipyridines for the construction of a series of 6,6′-bis(alkylamino)-2,2′-bipyridines (example pictured).
- Revisiting the Chichibabin Reaction: C2 Amination of Pyridines with a NaH–Iodide Composite,
Jia Hao Pang, Atsushi Kaga, Sven Roediger, Min Htoo Lin, Shunsuke Chiba,
Asian J. Org. Chem. 2019.
https://doi.org/10.1002/ajoc.201900094
Reference
- [1] New reaction for compounds containing the pyridine nucleus,
A. E. Chichibabin, O. A. Zeide,
J. Russ. Phys. Chem. Soc. 1914, 46, 1216.


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
