Fluorine is the most reactive chemical element. It is very toxic but used in many applications. The crystal structures of the allotropes α‐ and β‐F2 have each only been determined once using X-ray diffraction. This is because solid F2 is still extremely reactive. During the measurements, the element reacted with the copper sample holder and caused several explosions. The low signal intensity also made the interpretation of the measurements difficult.
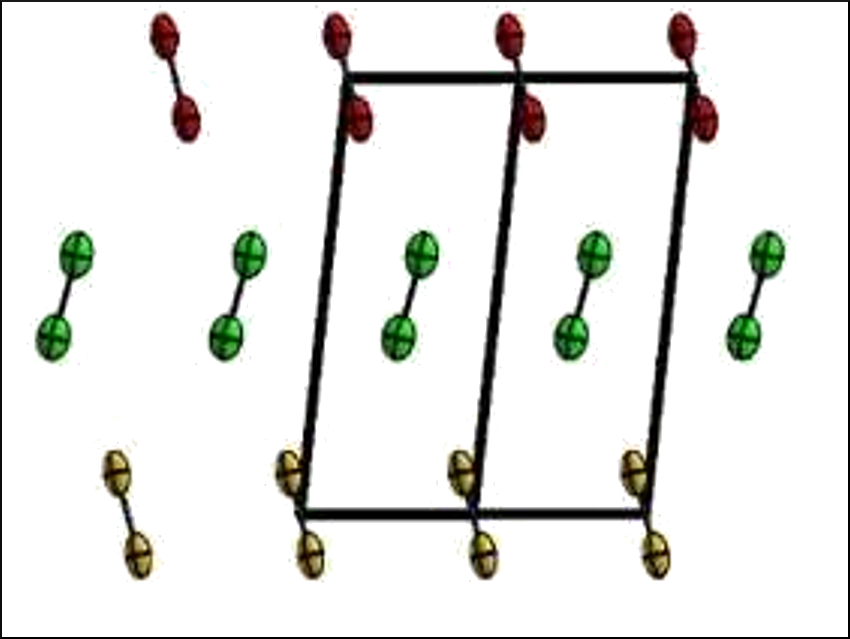
Florian Kraus, University of Marburg, Germany, and colleagues have accurately determined the atomic distances in the crystal structure of solid fluorine (structure of α‐F2 pictured). They investigated the structure of the element using neutrons at the research neutron source “Forschungsreaktor München II” (FRM II) of the Technical University Munich (TUM), Germany. Neutrons are particularly well suited to precisely locate fluorine atoms and can penetrate thick-walled sample containers.
According to Kraus, the team has resolved the atomic distance 70 % more accurately than the existing measurements. They found that α‐F2 crystallizes in the space group C2/c, with four F2 molecules in the unit cell and an F−F bond length of 1.404 Å. β‐F2 crystallizes in the space group Pm3n.
- The Crystal Structures of α‐ and β‐F2 Revisited,
Sergei I. Ivlev, Antti J. Karttunen, Markus Hoelzel, Matthias Conrad, Florian Kraus,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201805298