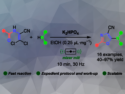
Dibenzo[hi,st]ovalene (DBOV, pictured) is a polycyclic aromatic hydrocarbon (PAH) consisting of 38 sp2-hybridized carbon atoms. DBOV can also be regarded as an atomically precise fragment of graphene. It is highly stable and shows strong red fluorescence. Thus, it has potential for applications in light-emitting devices. However, the introduction of functional groups to DBOV has been challenging.
Klaus Müllen, Max Planck Institute for Polymer Research, Mainz, and University of Mainz, both Germany, Akimitsu Narita, Max Planck Institute for Polymer Research and Okinawa Institute of Science and Technology Graduate University, Japan, and colleagues have developed a regioselective bromination of the 3- and 11-positions of DBOV. The team found that the bromination proceeded in a good yield of 79 % when they treated a DBOV derivative with two mesityl groups at the 6- and 14-positions with N-bromosuccinimide in tetrahydrofuran at room temperature.
The team also achieved further functionalization with either different aryl groups or triisopropyl (TIPS)-protected ethynyl groups by Suzuki or Sonogashira coupling reactions, respectively. These subsequent coupling reactions were performed under standard conditions. DBOV with 4-tert-butylphenyl groups showed an enhanced photoluminescence quantum yield of 97 % in solution. According to the researchers, they are using this functionalization method, for example, to make water-soluble DBOV for bioimaging applications.
- Regioselective Bromination and Functionalization of Dibenzo[hi,st]ovalene as Highly Luminescent Nanographene with Zigzag Edges,
Qiang Chen, Di Wang, Martin Baumgarten, Dieter Schollmeyer, Klaus Müllen, Akimitsu Narita,
Chem. Asian J. 2019.
https://doi.org/10.1002/asia.201801822
![Regioselective Functionalization of Dibenzo[hi,st]ovalene](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/1696d92b1fc.jpg)