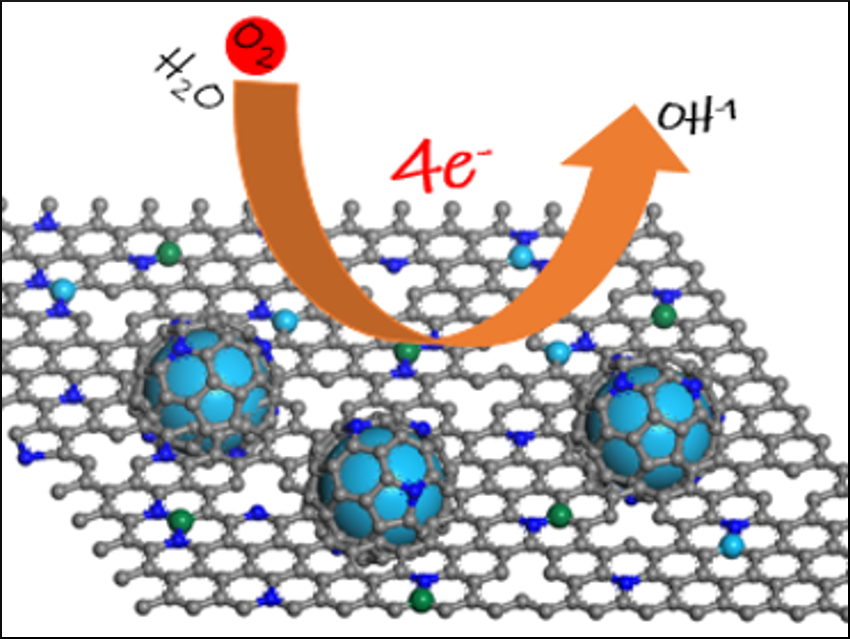
Metal-organic-framework (MOF)-derived nanocarbon materials codoped with iron or cobalt as well as nitrogen are promising for the cathodic oxygen reduction reaction (ORR) in fuel cells. They could replace expensive Pt/C catalysts. Due to the synergistic interactions between two active metal sites, Fe- and Co-codoped nanocarbon electrocatalysts can further enhance the oxygen reduction reaction (ORR) performance compared with their single-metal-doped counterparts.
Yue Zhao, Nanjing University, China, Hai‐Bin Zhu, Southeast University, Nanjing, China, and colleagues have prepared an Fe- and Co-codoped carbon electrocatalyst (Fe-Co-N-C-900), which can be used for oxygen reduction. The catalyst was synthesized from a CoII-based MOF, which was modified by anion exchange with potassium ferricyanide, followed by pyrolysis (pictured below).
A significant enhancement in ORR performance was observed in Fe-Co-N-C-900 over Co-N-C-900 because of the presence of multiple active sites (Fe-Nx, Co-Nx, N-Cx, and Co nanoparticles that are encapsulated in the graphitic carbon layer). This provides a new approach toward Fe- and Co-codoped nanocarbon electrocatalysts for oxygen reduction.

- Efficient Fe-Co-N-C Electrocatalyst Towards Oxygen Reduction Derived from a Cationic Co(II)-based Metal–Organic Framework Modified by Anion-Exchange with Potassium Ferricyanide,
Xiang-Lan Chen, Jia-Wei Huang, Yi-Chen Huang, Jie Du, Yu-Fei Jiang, Yue Zhao, Hai-Bin Zhu, Xian-Lan Chen,
Chem. Asian J. 2019.
https://doi.org/10.1002/asia.201801776