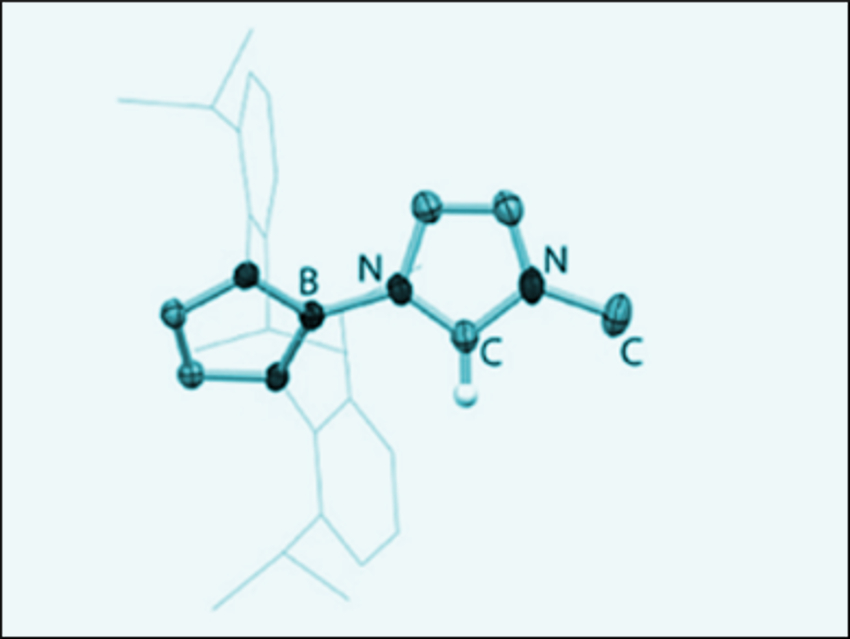
Singlet carbenes are useful as ligands in metal catalysts and for the stabilization of unusual main group compounds. Simon Aldridge and colleagues, Oxford University, UK, have tried to use the bulky, strongly donating diazaborolyl group {(HCDippN)2B} (Dipp = 2,6-iPr2C6H3) as the N-bound substituent of N-heterocyclic carbenes (NHCs). The imidazolium salts which acts as NHC precursors were synthesized using the bromoborane {(HCDippN)2}BBr and the parent heterocycles, imidazole or benzamidazole.
An N-to-C migration of the boryl substituent prevented the isolation of an NHC with two N-bound boryl groups. Kinetic and DFT studies on the migration process suggest an intramolecular 1,2-shift. In contrast to earlier reports, it seems that such shifts are feasible in aromatic carbenes.
NHCs with a single N-bound boryl group and one methyl substituent (example pictured) could be accessed. These NHCs were characterized by NMR spectroscopy and could be trapped by metal fragments. The synthesized carbenes provide an increased electron donor ability compared with unsaturated NHCs and a large (if unsymmetrical) steric bulk. Boryl substituted carbenes are not common and, according to the team, these are the first examples of NHCs featuring an exocyclic N-boryl group.
- Borylated N-heterocyclic carbenes: rearrangement and chemical trapping,
Simon Aldridge, Lilja Kristinsdottir, Petra Vasko, Haoyu Niu, Eugene Kolychev, Jesus Campos, Maria Angeles Fuentes, Jamie Hicks, Amber L Thompson,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201804808