In photosynthesis, the oxygen-evolving complex in photosystem II uses a water-splitting catalyst to split water into protons, electrons, and dioxygen molecules. This Mn4Ca cluster (pictured left below) is a blueprint for man-made water-splitting catalysts. However, the cluster is prone to decomposition and it is, thus, challenging to mimic the structure and function of the biological catalyst in a laboratory setting.
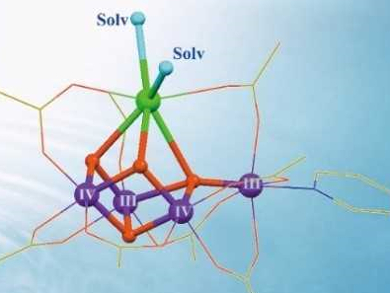
Chunxi Zhang and colleagues, Chinese Academy of Sciences, Beijing, have synthesized two robust artificial Mn4Ca complexes (pictured right below). The calcium sites are stabilized by exchangeable solvent molecules, i.e., acetonitrile (CH3CN) or N,N-dimethylformamide (DMF). The solvent molecules were introduced by recrystallization from solutions containing the respective solvent. The complexes closely mimic the core geometric and electronic structures of the natural catalyst contained in photosystem II.
These artificial complexes could provide important chemical clues to understand the structure and mechanism of their biological counterpart. They may lead to development of more efficient catalysts for artificial photosynthesis.

- Artificial Mn4Ca-cluster with Exchangeable Solvent Molecules Mimicking the Oxygen-Evolving Center in Photosynthesis,
Changhui Chen, Yang Chen, Ruoqing Yao, Yanxi Li, Chunxi Zhang,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201814440