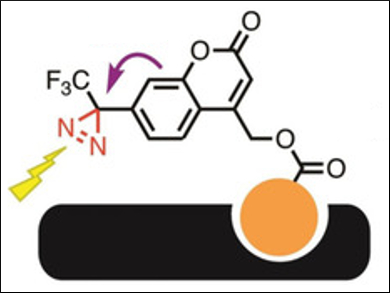
Takenori Tomohiro, University of Toyama, Japan, and colleagues have developed a compact diazirine-based photolabeling agent for proteins, based on a (coumarin-4-yl)methyl ester scaffold, i.e., (5-ethoxy-2-oxo-7-(3-(trifluoromethyl)-3H-diazirin-3-yl)-2H-chromen-4-yl)methyl ester (pictured above). A photoinduced electron transfer (PeT) between diazirine and coumarin quenches the coumarin’s fluorescence.
The labeling agent binds to a target protein using a specific ligand (pictured in orange). It can then be activated with light at 365 nm, which causes the photolysis of the diazirine group (see below, pictured in red) and creates a covalent bond between probe and protein. At the same time, the fluorescence of the coumarin is activated by the removal of the diazirine quencher.
The bond between the (coumarin‐4‐yl)methyl ester and the protein-targeting ligand can then be cleaved using light at 313 nm, leaving the fluorescent marker on the protein. The labeled protein can then be selectively isolated. The coumarin tag is small and stable, which makes a subsequent liquid chromatography–mass spectrometry (LC‐MS)‐based protein identification possible.

- Photoinduced Electron Transfer-Regulated Protein Labeling With a Coumarin-Based Multifunctional Photocrosslinker,
Yusuke Hotta, Tsukasa Kaneko, Ryuji Hayashi, Akito Yamamoto, Shota Morimoto, Junya Chiba, Takenori Tomohiro,
Chem. Asian J. 2019.
https://doi.org/10.1002/asia.201801673