Electrochemical water splitting to produce hydrogen could be an important part of sustainable fuel generation in the future. Molybdenum diselenide (MoSe2) is a promising nonprecious-metal-based electrocatalyst for the hydrogen evolution reaction (HER) in acidic media. However, it has inferior HER catalytic kinetics in alkaline solutions, mainly due to the sluggish water adsorption/dissociation process.
Wenping Sun, Haibo Yu, University of Wollongong, Australia, and colleagues have found that heteroatom-doped MoSe2 nanosheets can be used as highly active electrocatalysts for HER in alkaline media. The nanosheets were synthesized using a simple, one‐step hydrothermal method. A series of Ni‐doped MoSe2 nanosheets (NixMo1−xSe2, x = 0.05, 0.1, 0.15, 0.2) were obtained by varying the molar ratio of Ni and Mo.
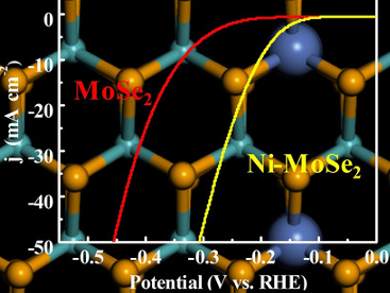
The Ni-doped MoSe2 nanosheets have a high catalytic activity, a low overpotential, and a large exchange current density. Density functional theory (DFT) calculations show that Ni doping plays a key role in facilitating water adsorption and optimizing hydrogen adsorption. According to the team, the work could help with the development of low-cost and efficient electrocatalysts for alkaline water splitting.
- Heteroatom-doped MoSe2 Nanosheets with Enhanced Hydrogen Evolution Kinetics for Alkaline Water Splitting,
Guoqiang Zhao, Xingyong Wang, Shaolan Wang, Kun Rui, Yaping Chen, Haibo Yu, Jing Ma, Shi Xue Dou, Wenping Sun,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201801645