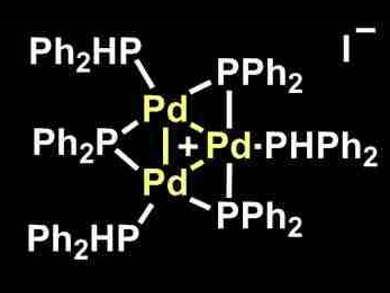
Monomeric transition-metal complexes are often used in homogeneous catalysis. The synergistic effects in complexes with multiple metal atoms could enable new reactivities and selectivities.
Franziska Schoenebeck, RWTH Aachen University, Germany, and colleagues have studied the reactivity of an overall cationic Pd trimer complex (pictured). The Pd trimer selectively reacts with aryl iodides, whereas aryl bromides and chlorides remain untouched in its presence. This chemoselective activation of C–I bonds is in stark contrast to the reactivity of common Pd(0) monomeric catalysts. Translated into catalysis, the team was able to perform C–I-selective cross-coupling reactions of aryl iodides with Grignard reagents in the presence of C–Br and C–Cl groups.
This operationally simple methodology allows for both arylation and alkylation reactions and tolerates steric hindrance at both coupling partners. The protocol works equally well with an in situ-formed Pd trimer by simply pre-stirring a mixture of a commercially available Pd(I)–Pd(I) dimer with diphenylphosphine for 10 min at room temperature before adding the coupling partners.
- C–I Selective Cross-Coupling Enabled by a Cationic Pd Trimer,
Claudia J. Diehl, Thomas Scattolin, Ulli Englert, Franziska Schoenebeck,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201811380