Enzymes could potentially be used to efficiently produce compounds such as building blocks for polymers or pharmaceuticals. Enzymes can catalyze reactions in a specific and efficient manner and can be used as alternatives to some chemical processes. However, the use of enzymes requires, e.g., sufficient enzyme stability, efficient cofactor regeneration, and easy production of the enzymes.
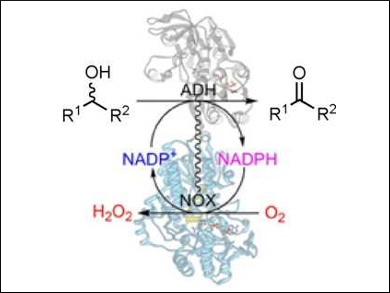
Friso Aalbers and Marco Fraaije, University of Groningen, The Netherlands, have designed an artificial enzyme by fusing two different types of enzymes, an alcohol dehydrogenase (ADH) and an NADPH oxidase (NOX). The fusion enzymes were produced in E. coli cells.
ADHs typically catalyze reactions that use oxidized nicotinamide adenine dinucleotide phosphate (NADP+) as an electron acceptor and transform alcohols into aldehydes or ketones. Inversely, the enzymes can also catalyze ketone reduction reactions by using a reduced nicotinamide cofactor. Combing the two enzymes ensures a rapid regeneration of the required nicotinamide cofactor.
The bifunctional fusion enzymes were found to be efficient biocatalysts to perform alcohol oxidations, merely requiring a catalytic amount of cofactor and molecular oxygen. By fusing these redox enzymes, the production and use of the two enzymes is greatly simplified.
- Design of Artificial Alcohol Oxidases: Alcohol Dehydrogenase-NADPH Oxidase Fusions for Continuous Oxidations,
Friso S. Aalbers, Marco W. Fraaije,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800421