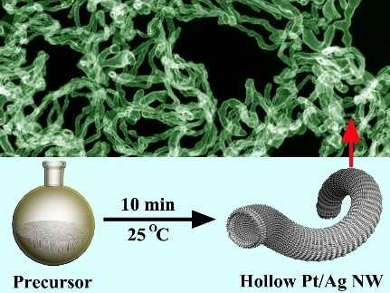
Hollow platinum-based nanostructures can maximize the efficiency of use of the expensive metal, especially in catalysis. However, this generally requires complicated multistep template methods. This is due to the fact that the reduction of Pt(IV) or Pt(II) to Pt is usually faster than the formation of a template. This hampers the large-scale application of such hollow Pt nanostructures.
Yun Yang, Wenzhou University, China, Shaoming Huang, Wenzhou University and Guangdong University of Technology, China, and colleagues have developed a simple one-step method for preparing hollow Pt/Ag nanowires. The method is based on simply tuning the pH value of the reaction mixture. A pH value of 11 makes the reduction of Pt(IV) to Pt significantly slower than that of Ag(I) to Ag. This allows the preferential formation of Ag nanowire templates, which then transform into hollow Pt/Ag nanostructures (pictured) after a galvanic reaction with Pt(IV).
The prepared Pt/Ag nanostructures have a higher activity in the electrochemical oxidization of methanol than the commonly used commercial Pt/C catalyst. According to the researchers, a large‐scale preparation of the nanocatalyst should be easy to achieve using this method.
- Tuning Ion Complexing To Rapidly Prepare Hollow Ag-Pt Nanowires with High Activity toward the Methanol Oxidization Reaction,
Fangyan Liu, Peimei Qi, Xiaoli Liang, Wei Chen, Benxia Li, Lijie Zhang, Yun Yang, Shaoming Huang,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201804250