Global ammonia production from dinitrogen happens via two dominating processes: the natural enzymatic process catalyzed by nitrogenase, which occurs at ambient temperature and atmospheric pressure, and the industrial Haber-Bosch process, which occurs at high temperatures and pressures. This latter process currently consumes approximately 1 % of the world’s total energy per year. Lower energy routes to NH3 synthesis would be useful.
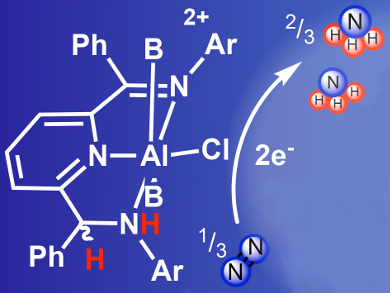
Louise A. Berben and colleagues, University of California, Davis, USA, have developed a transition-metal-free electrochemical reduction of N2 into NH3, using an aluminium complex (pictured in white). This complex facilitates NH3 production at a low reduction potential of –1.2 V measured vs. a saturated calomel electrode (SCE).
The reaction occurs under 1 atm of N2 when the twice-protonated Al(III) complex is reduced by two electrons (pictured). Sub-stoichiometric quantities of NH3 are generated. This work provides new ideas for strategies, reaction mechanisms, and catalysts derived from main group elements and redox active ligands which could be used to mediate small-molecule transformations.
- Electrochemical Reduction of N2 to NH3 at Low Potential by a Molecular Aluminum Complex,
Tobias J. Sherbow, Emily J. Thompson, Amela Arnold, Richard I. Sayler, R. David Britt, Louise A. Berben,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201804454