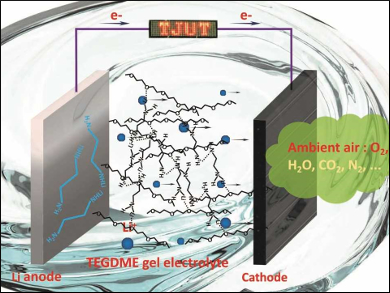
Flexible lithium-air batteries (LABs) are a promising power source for wearable electronic devices because of their high theoretical energy density. However, the application of LABs is limited by lithium anode passivation, huge interface resistance when the anode is bent, poor cycle life, and leakage of liquid electrolytes.
Yonggang Wang, Fudan University, Shanghai, China, Yi Ding and Xizheng Liu, Tianjin University of Technology, China, and colleagues have developed a flexible LAB which contains a tetraethylene glycol dimethyl ether (TEGDME) gel electrolyte. The gel electrolyte is formed in situ in an operational battery by a direct liquid TEGDME cross-linking reaction with lithium ethylenediamine (LiEDA) on the lithium anode.
A battery assembled from a LiEDA-modified lithium anode, liquid electrolyte, and a manganese oxide/carbon cloth cathode (pictured) can be used to light up a light-emitting diode (LED) display. The high ionic conductivity of the gel electrolyte reduces corrosion of the lithium anode. This allows a battery cycle life of more than 1175 hours in ambient air. The in-situ fabrication of the electrolyte promotes interfacial contact with both electrodes and ensures that the LAB is robust and flexible.
- Flexible Lithium-Air Battery in Ambient Air with an In Situ Formed Gel Electrolyte,
Xiaofeng Lei, Xizheng Liu, Wenqing Ma, Zhen Cao, Yonggang Wang, Yi Ding,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201810882