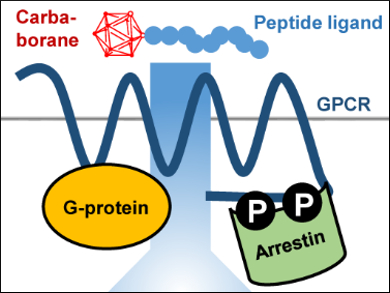
G-protein coupled receptors are transmembrane proteins that convert extracellular into intracellular signals. Thay are, therefore, useful pharmaceutical targets. Exploring the structural changes that are associated with this signal transduction requires receptor-selective ligands.
Kathrin Bellmann-Sickert, Leipzig University, Germany and colleagues have identified a shortened peptide ligand which is selective for the human Y1 receptor (hY1R), a G‐protein coupled receptor. The peptide is based on a truncated version of neuropeptide Y and carries a carbaborane cluster. It fully activates the receptor, while the same peptide without the carbaborane cluster is only partially active.
The group studied the role of the carbaborane using structural analogues of the cluster and investigated two different signaling pathways that can be triggered by hY1R activation: G-protein and arrestin signaling. The team found that hydrophobicity at the position of the carbaborane cluster is the driving force for G-protein activation. For arrestin signaling, the bulky nature of the carbaborane cluster is important. This points to a specific binding pocket in the receptor which is responsible for arrestin recruitment.
These findings, together with the facile simple synthesis of the short peptides, encourage the creation of a toolbox of carbaboranylated peptide ligands. Such ligands could stabilize different receptor conformations with varying activities and be used for structural receptor investigations.
- Carbaboranylation of Truncated C-Terminal Neuropeptide Y Analogue Leads to Full hY1 Receptor Agonism,
Sven Hofmann, Josephin Lindner, Annette G. Beck-Sickinger, Evamarie Hey-Hawkins, Kathrin Bellmann-Sickert,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800343