The red rose is the traditional Valentine’s Day flower. Let’s look at the chemistry behind its color and its aroma.
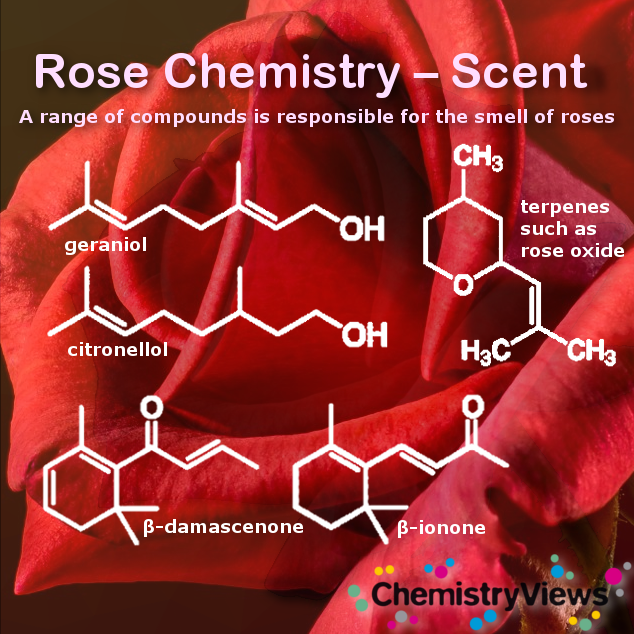
Rose Scent
The scent in roses comes from one or more of over 300 compounds. These volatile compounds are picked up by our nose receptors. Each of these compounds evaporates at a different rate. Therefore, a rose fragrance will change over time. Clove evaporates, for example, much more slowly than citrus. So we pick up a citrus scent that disappears with the clove scent appearing at our nose receptors.
Rose scent may be more powerful with the first blooms of summer and early in the morning. Also, the cutting can change the chemical releases that we smell. A rose not very fragrant outdoors may be quite scented once in a vase inside.
Rose oil is expensive. To produce 1 kg, about 10 tonnes of petals will be needed. This means that the majority of perfumes are produced with synthetic alternatives. Most true rose oil comes from Bulgaria, Morocco, Iran, Turkey, and more recently from China. The oil is extracted from the petals, either with alcohol or through distillation.
Rose Scent in Clothing
Rudolf Hufenus and colleagues, Laboratory of Advanced Fibers, Empa, St. Gallen, Switzerland, have used a continuous melt-spinning process to produce so-called liquid-core fibers. These are fibers filled with, for example, flowery rose scent.
Whereas thus far, it has only been possible to fill hollow fibers of limited length in a complex two-step process, with this process, longer fibers that are being filled during production can be produced. These bi-component fibers can then be processed into textiles. The fragrances are released slowly and continuously through the gas-permeable covering of the polymer fibers so that the textiles have a fragrant effect over numerous washing cycles.
References
 |
Red Rose Pigments |
 |
The Aroma of Roses |
- Melt-spun polymer fibers with liquid core exhibit enhanced mechanical damping,
Rudolf Hufenus, Laura Gottardo, A. Andrés Leal, Armin Zemp, Kurt Heutschi, Philipp Schuetz, Veronika R. Meyer, Manfred Heuberger,
Materials & Design 2016, 110, 685–692.
https://doi.org/10.1016/j.matdes.2016.08.042