Biological processes in cells can be made visible using fluorescent and bioluminescent proteins. However, their efficacy in larger organisms is limited by the poor light penetration through living tissue. Proteins observable by magnetic resonance imaging (MRI) overcome this limitation. However, conventional 1H MRI is characterized by low sensitivity. The use of hyperpolarized 129Xe instead of 1H allows the detection of proteins at physiological concentrations. Proteins that bind 129Xe are a new class of MRI contrast agents.
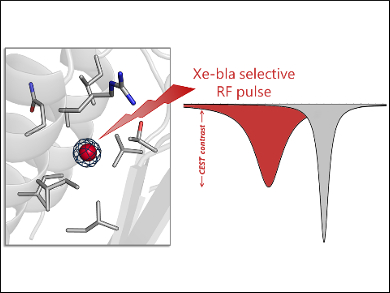
Ivan J. Dmochowski, University of Pennsylvania, Philadelphia, USA, and colleagues have studied one of these proteins, namely, TEM-1 β-lactamase (bla). The team used X-ray crystallography and site-directed mutagenesis (i.e., the introduction of DNA mutations) to identify the specific Xe-binding site in bla responsible for its unique MRI signal. The researchers also modeled the entry and exit of Xe to and from this binding site using molecular dynamics (MD) simulations.
Crystal structures of bla derivatized with Xe showed a high‐occupancy Xe atom bound in a hydrophobic allosteric site, as well as low‐occupancy Xe bound at two additional sites. Mutagenesis studies confirmed that the high-occupancy Xe site is responsible for the MRI contrast. These insights into the Xe–protein interaction can be used to guide the design of bla variants with improved detection sensitivities.
- A Structural Basis for 129Xe Hyper-CEST Signal in TEM-1 β-Lactamase,
Benjamin W. Roose, Serge D. Zemerov, Yanfei Wang, Marina A. Kasimova, Vincenzo Carnevale, Ivan J. Dmochowski,
ChemPhysChem 2018.
https://doi.org/10.1002/cphc.201800624