Polyfunctionalized arenes are commonly found in pharmaceuticals, materials, and natural products. Pd(0)-catalyzed functionalizations of poly(pseudo)halogenated arenes could be one approach. However, these reactions often show an unpredictable chemoselectivity.
Franziska Schoenebeck, RWTH Aachen University, Germany, and colleagues have developed the first rapid (<10 minutes), general, and air-tolerant method to selectively functionalize C–OTf, C–Cl, or C–Br groups using a Negishi cross-coupling strategy (OTf = SO3CF3). The team used an air‐ and moisture‐stable Pd(I) dimer (pictured below) as the catalyst and N‐methyl‐2‐pyrrolidinone (NMP) as the polar solvent. The Pd(I) dimer is converted to an “ate” complex in the solvent. This changes the catalyst’s reactivity and influences the preference of functionalization of the different (pseudo)halogen groups.
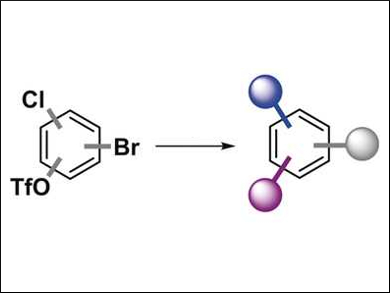
This methodology is tolerant to numerous functional groups and allows both arylations and the generally more challenging alkylations. The approach allowed the team to perform the first triply selective, sequential C–C coupling in under 40 minutes, first at C–Br groups, then at C–OTf groups, and finally at C–Cl groups (pictured below). The reactions use the same catalyst for all three steps. The method is operationally simple and could lead to a modular and programmable synthesis of densely functionalized arenes.

- Modular Functionalization of Arenes in a Triply Selective Sequence: Rapid C(sp2) and C(sp3) Coupling of C–Br, C–OTf, and C–Cl Bonds Enabled by a Single Palladium(I) Dimer,
Sinead T. Keaveney, Gourab Kundu, Franziska Schoenebeck,
Angew. Chem. Int. Ed. 2018, 57, 12573–12577.
https://doi.org/10.1002/anie.201808386