Self-assembled porphyrin capsules or cages are interesting materials in the field of supramolecular chemistry. There are covalently connected (metallo)porphyrin units, coordination‐driven self‐assembled porphyrins, and even bis-porphyrin cages held together by mechanical bonds. However, examples of self-assembled porphyrin capsules stabilized through hydrogen bonding are relatively rare.
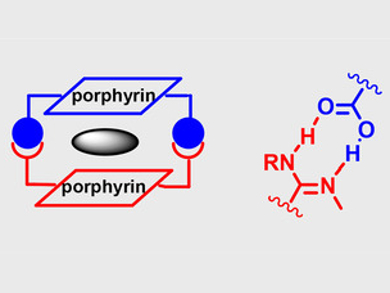
Yuji Tokunaga, University of Fukui, Japan, and colleagues have developed a self-assembled heterodimer of a pair of different porphyrin units (pictured below), stabilized through two amidinium–carboxylate bridges (pictured above on the right). Equimolar amounts of the porphyrins were combined in chloroform to give the heterodimer.
The dimer binds electron-deficient π-aromatic compounds, forming three-layer π-aromatic structures (pictured above on the left). The rates of association and dissociation depend on the electron-deficiency of the aromatic guest molecules. Since the dimer is asymmetrical, interesting phenomena such as orientational isomerism could occur upon the binding of guest molecules.

- Recognition Behavior of a Porphyrin Heterodimer Self-Assembled through an Amidinium-Carboxylate Salt Bridge,
Masaki Kimura, Jyunichi Miyashita, Shinobu Miyagawa, Tsuneomi Kawasaki, Hikaru Takaya, Yuji Tokunaga,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800382