Sodium- and lithium-sulfur batteries have a low cost and high charge-storage capacity. In lithium-sulfur batteries, sulfur is reduced during discharge to form lithium sulfide. Both sulfur and lithium sulfide are insulating. Thus, the electrodes used are typically (nano-)composites of sulfur and conductive carbon.
Philipp Adelhelm, University of Jena, Germany, and colleagues have found a phenomenon called “sulfur spillover”, i.e., the infiltration of carbon by sulfur. This could have important consequences for the preparation of carbon-sulfur nanocomposites and their behavior during battery operation.
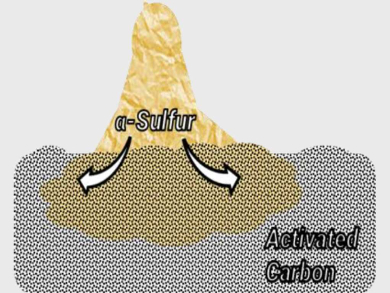
In a mixture of S8 and porous carbon, the team observed that sulfur is rapidly redistributed on the carbon surface at room temperature (process pictured). Within days, sulfur completely covers the carbon surface. The bulk properties of sulfur, such as its crystallinity and melting point, were no longer observed after six days because of the loss of the sulfur’s original structure. The researchers used X‐ray diffraction, thermal analysis, elemental analysis, Raman spectroscopy, and electron microscopy to monitor this phenomenon.
The extent of the phenomenon depends on the surface area—or spillover capacity—of the carbon material used, and declines at low temperatures. As much as 50 wt% of the sulfur undergoes spillover on highly porous carbon. The researchers believe that sulfur spillover may occur continuously during cell cycling of metal-sulfur batteries, which is useful information for the development of improved batteries.
- Sulfur Spillover on carbon materials and possible impacts on metal-sulfur batteries,
Philipp Adelhelm, Lukas Medenbach, Ines Escher, Linda Zedler, Benjamin Dietzek, Marc Armbrüster, Nicolas Köwitsch,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201807295