Photolabile protecting groups or “photocages” can be used to protect, e.g., alcohols. They can be removed by irradiation with light.
Alexander Heckel, Irene Burghardt, University of Frankfurt am Main, Germany, and colleagues have used the simple 3-diethylaminobenzyl (DEAMb) photocage as a starting point for the synthesis of improved photocages. The main drawback of DEAMb is the need to use harsh UV light (λ < 350 nm) for cleavage.
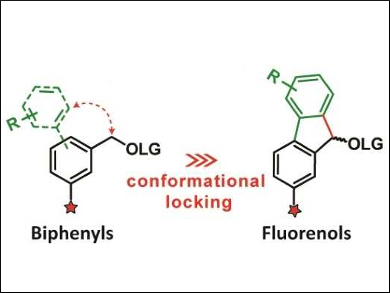
The team first extended the π‐conjugation in the molecule by including a second aryl group, leading to biphenyl derivatives (pictured left, LG = leaving group). This causes a redshift of the group’s light absorbance. However, the phenyl rings in these biphenyl compounds are twisted out of coplanarity, which limits the π‐orbital overlap. Thus, the researchers used a more rigid, planar fluorene structure with improved π‐conjugation (pictured right). The absorbance bands of these compounds extend almost into the visible spectral range.
Overall, the team found that a second aromatic ring can improve properties of photolabile protecting groups. Fluorenes provide better molar absorption coefficients than the less rigid biphenyls. The photochemical properties of these fluorene derivatives can easily be tuned by changing the substituents.
- A New Photocage Derived from Fluorene,
Matiss Reinfelds, Jan von Cosel, Konstantin Falahati, Carsten Hamerla, Tomáš Slanina, Irene Burghardt, Alexander Heckel,
Chem. Eur. J. 2018, 24, 13026–13035.
https://doi.org/10.1002/chem.201802390


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
